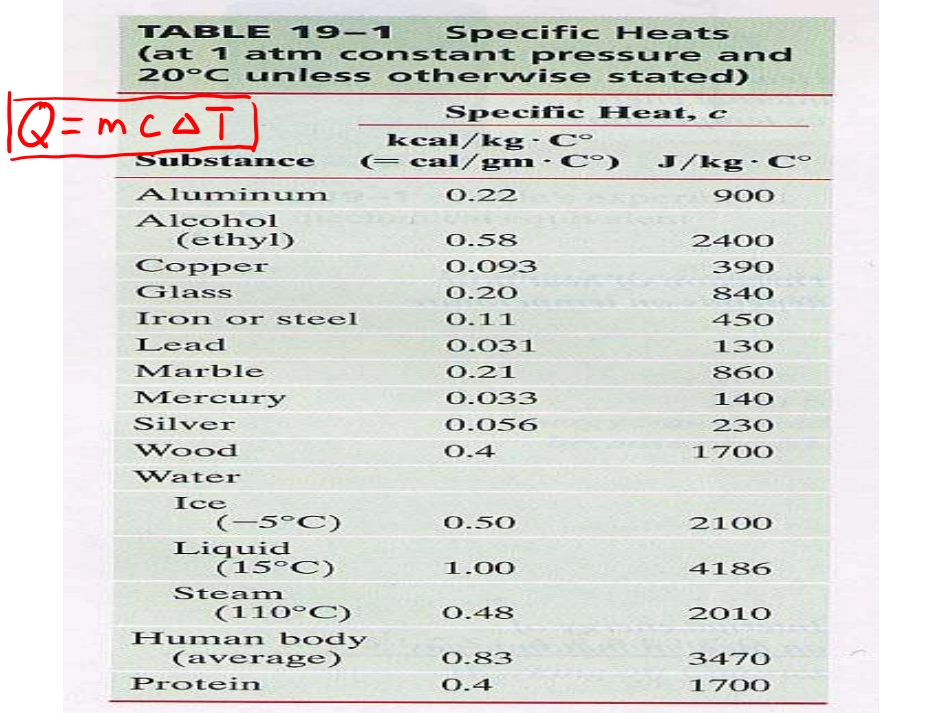
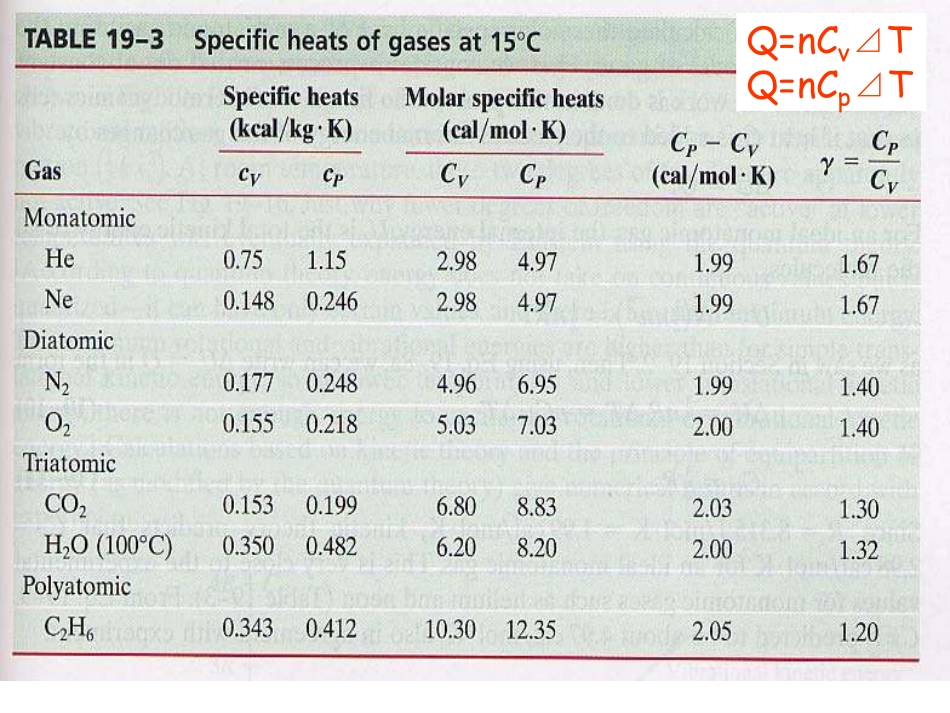
2021/9/51大学物理(热学)1)Review2)Carnot’sIdeas3)TheSecondLaw4)Carnot’scycle2006-12-13Q=nCv⊿TQ=nCp⊿TPV=CPV=C瓦特J.Watt蒸汽机用热做功——存在限制否?卡诺SadiCarnot卡诺循环(1824)“Theproductionofmotivepoweristhendueinsteam-enginesnottoanactualconsumptionofcaloric,buttoitstransportationfromawarmbodytoacoldbody,thatis,toitsre-establishmentofequilibrium-anequilibriumconsideredasdestroyedbyanycausewhatever,bychemicalactionsuchascombustion,orbyanyother.”Carnot’sideasCarnot’sideasQW“Accordingtothisprinciple,theproductionofheataloneisnotsufficienttogivebirthtotheimpellingpower:itisnecessarythatthereshouldalsobecold;withoutit,theheatwouldbeuseless.”Carn...