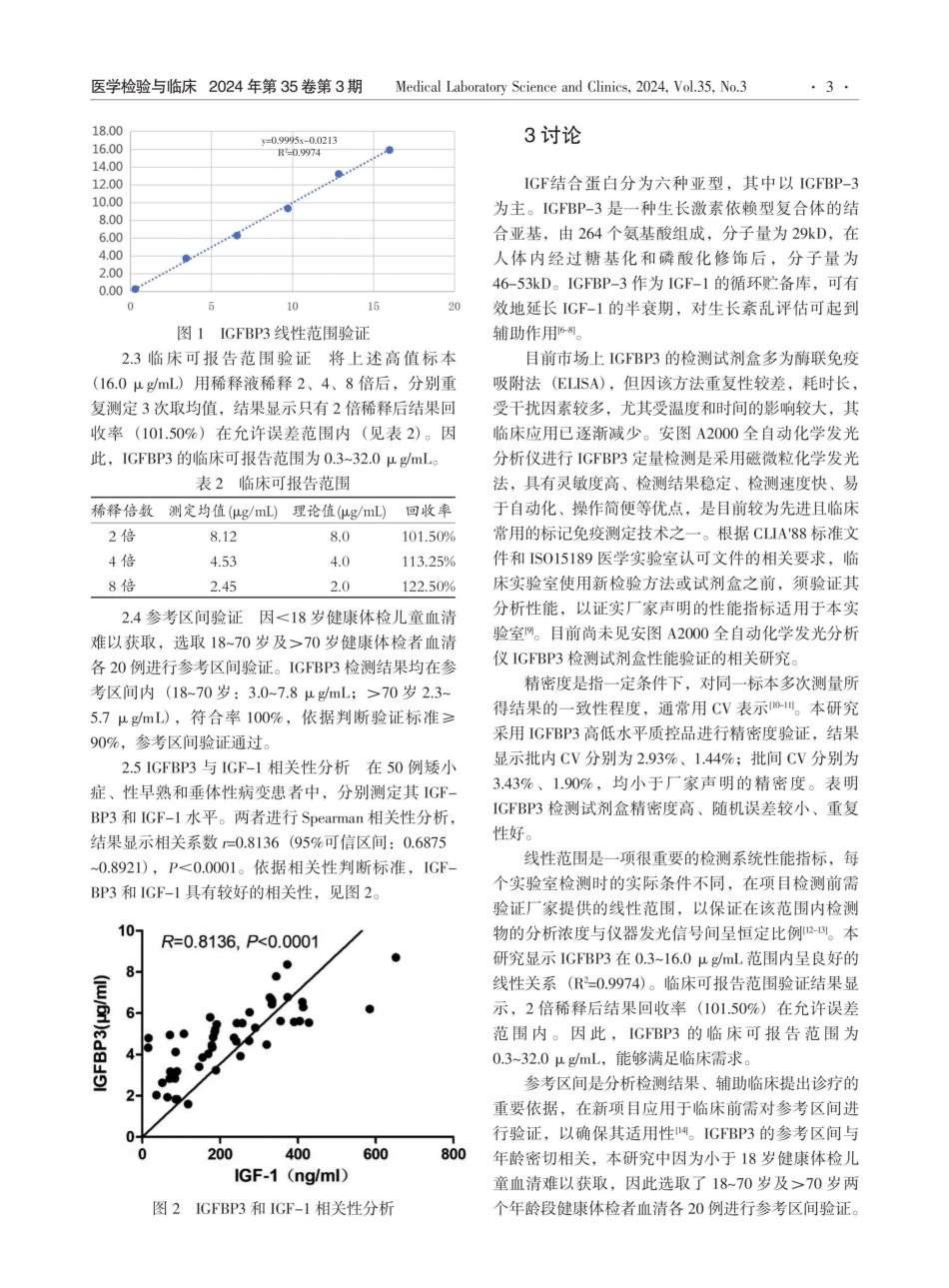
医学检验与临床2024年第35卷第3期MedicalLaboratoryScienceandClinics,2024,Vol.35,No.3doi:10.3969/j.issn.1673-5013.2024.03.001安图A2000全自动化学发光分析仪检测IGFBP3的性能验证及与IGF-1相关性研究-陈庆文刘英杰关郑桂喜(山东大学齐鲁医院检验科,山东济南250012)[摘要]目的:对安图A2000全自动化学发光仪检测血清胰岛素样生长因子结合蛋白3(IGFBP3)的性能进行评价,并探讨其在矮小症、性早熟及垂体性病变中与IGF-1的相关性。方法:对安图A2000系统检测IGFBP3的精密度(CV)、线性范围、可报告范围、参考区间进行验证。选取2023年1月一2023年9月在本院诊断或治疗的矮小症21例、性早熟10例、垂体性病变19例,回顾性分析其胰岛素生长因子一I(IGF-1)和IGHBP3水平,并进行Spearman相关性分析。结果:IGFBP3高低水平质控品批内CV为2.019%、2.931%,批间CV为3.818%、4.137%;测定结果在0.3~16.0μg/mL范围内呈线性,线性回归方程为Y=0.9995X~0.0213,R2=0.9974;临床可报告范围为0.3~32.0μg/mL;18~70岁和>70岁健康体检者检测结果均在厂家提供的参考区间内;IGFBP3和IGF-1在矮小症、性早熟和垂体性病变患者中具有较好的相关性(r=0.81,P<0.0001)。结论:安图A2000检测血清IGFBP3的性能能够满足实验室质量要求,且与IGF-1具有较好的相关性。[关键词】全自动化学发光分析仪;胰岛素样生长因子结合蛋白3;胰岛素样生长因子-1;性能验证TheperformanceofAutoA2000automaticchemiluminescenceanalyzerindetectionofIGFBP3anditscorrelationwithIGF-1CHENQing,LIUYing-jie,ZHENGGui-xi(DepartmentofclinicalLaboratory,QiluHospitalofShandongUniversity,ShandongJinan250012)[Abstract)Objective:Toevaluatetheperformanceofseruminsulin-likegrowthfactorbindingprotein3(IGFBP3)detectedbyAutoA2000automaticchemiluminescenceinstrument,andtoexplorethecorrelationbetweenIGFBP3andIGF-1inpatientswithshortstature,precociouspubertyandpituitarylesions.Methods:Theprecision(CV),linearrange,clinicalreportablerangeandreferenceintervalofIGFBP3detectedbyAutoA2000systemwereverified.Atotalof21casesofshortstature,10casesofprecocityand19casesofpituitarylesionsdiagnosedortreatedinourhospitalfromJanuary2023toSeptember2023wereselectedThelevelsofinsulingrowthfactor-I(IGF-1)andIGHBP3wereretrospectivelyanalyzed,andtheSpe...