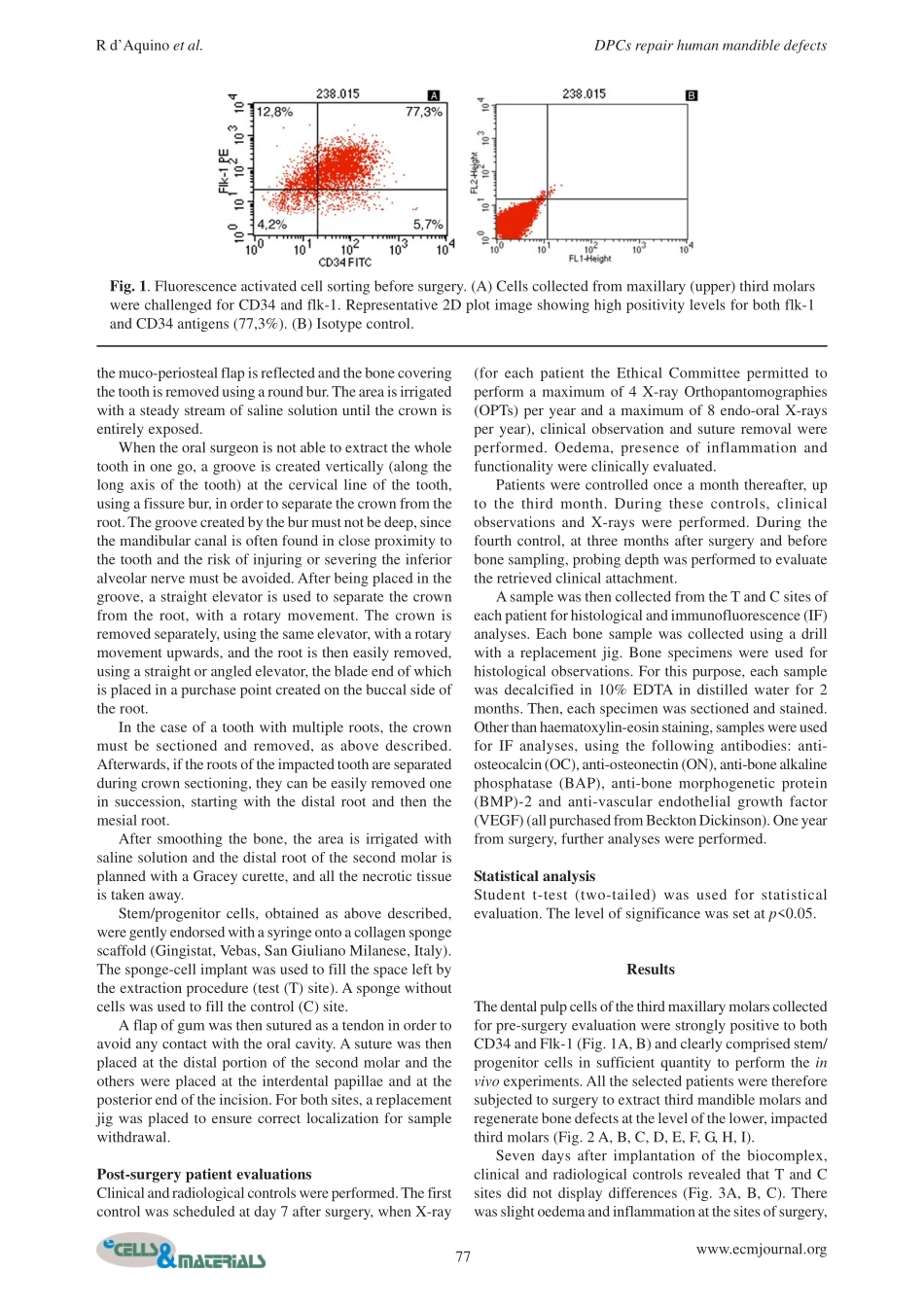
75www.ecmjournal.orgRd’Aquinoetal.DPCsrepairhumanmandibledefectsEuropeanCellsandMaterialsVol.182009(pages75-83)ISSN1473-2262AbstractInthisstudyweusedabiocomplexconstructedfromdentalpulpstem/progenitorcells(DPCs)andacollagenspongescaffoldfororo-maxillo-facial(OMF)bonetissuerepairinpatientsrequiringextractionoftheirthirdmolars.TheexperimentswerecarriedoutaccordingtoourInternalEthicalCommitteeGuidelinesandwritteninformedconsentwasobtainedfromthepatients.Thepatientspresentedwithbilateralbonereabsorptionofthealveolarridgedistaltothesecondmolarsecondarytoimpactionofthethirdmolaronthecorticalalveolarlamina,producingadefectwithoutwalls,ofatleast1.5cminheight.Thisclinicalconditiondoesnotpermitspontaneousbonerepairafterextractionofthethirdmolar,andeventuallyleadstolossalsooftheadjacentsecondmolar.MaxillarythirdmolarswereextractedfirstforDPCisolationandexpansion.Thecellswerethenseededontoacollagenspongescaffoldandtheobtainedbiocomplexwasusedtofillintheinjurysiteleftbyextractionofthemandibularthirdmolars.ThreemonthsafterautologousDPCgrafting,alveolarboneofpatientshadoptimalverticalrepairandcompleterestorationofperiodontaltissuebacktothesecondmolars,asassessedbyclinicalprobingandX-rays.Histologicalobservationsclearlydemonstratedthecompleteregenerationofboneattheinjurysite.Optimalboneregenerationwasevidentoneyearaftergrafting.ThisclinicalstudydemonstratesthataDPC/collagenspongebiocomplexcancompletelyrestorehumanmandiblebonedefectsandindicatesthatthiscellpopulationcouldbeusedfortherepairand/orregenerationoftissuesandorgans.Keywords:Dentalpulpstem/progenitorcells(DPCs),bone,humanmandible,stem/progenitorcellgraft,bioscaffold,regenerativemedicine,clinicalstudy.Addressforcorrespondence:GianpaoloPapaccioDepartmentofExperimentalMedicineSectionofHistologyandEmbryology,TERMDivision2ndUniversityofNaples,viaL.Armanni,5,80138Naples(Italy)TelephoneNumber:+39081-5666014FAXNumber:+39081-5666015Skype:StemfaceE-mail:gianpaolo.papaccio@unina2.itIntroductionTheaimoftissueengineering(TE)isthereg...