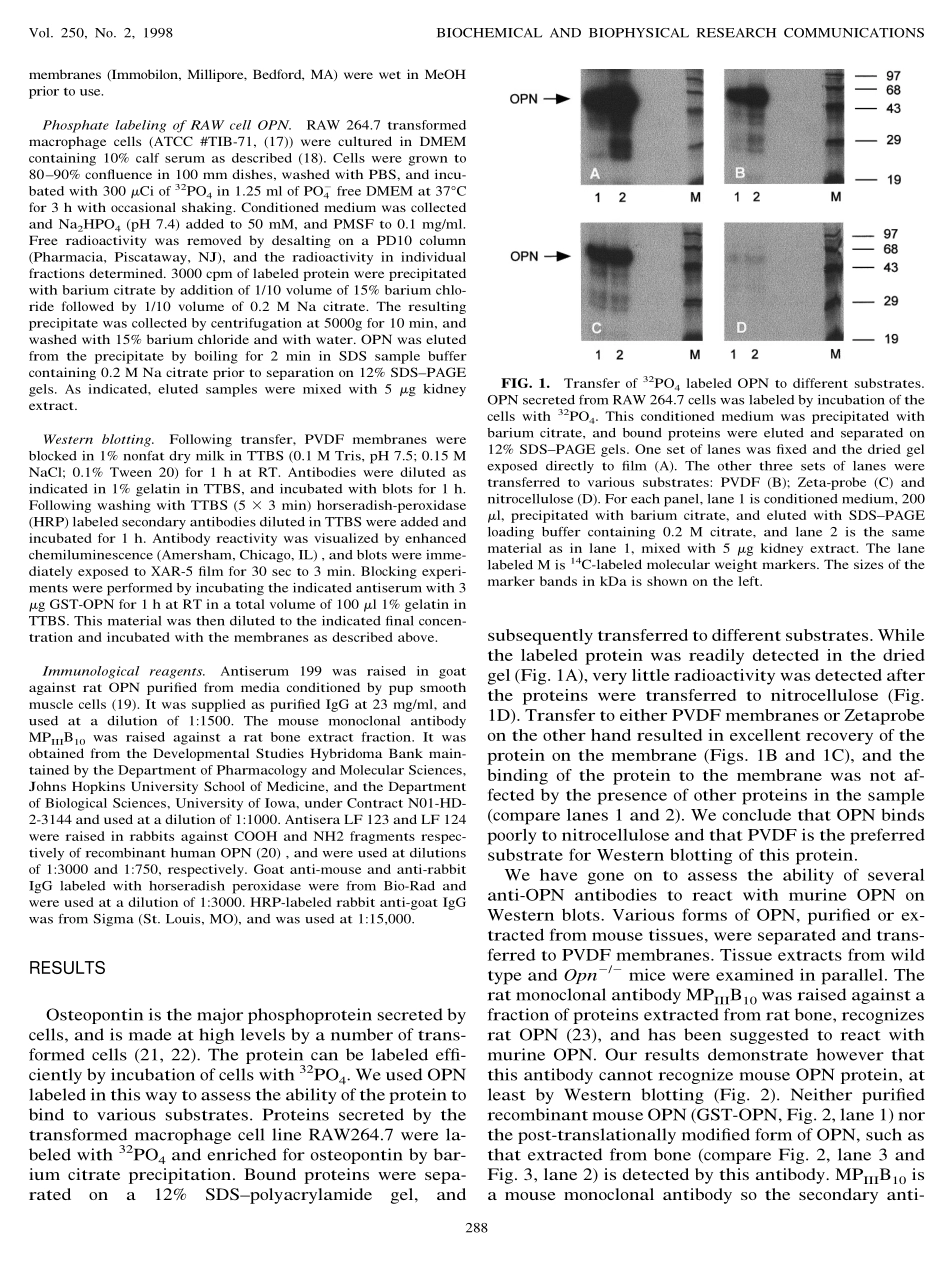
DetectionofMouseOsteopontinbyWesternBlottingSusanR.Rittling1andFeiFengDepartmentofCellBiologyandNeuroscience,RutgersUniversity,Piscataway,NewJersey08854ReceivedAugust14,1998Severaldifferentantibodiestomouseand/orratos-teopontinhavebeendeveloped,andsomeantiseraraisedagainsthumanosteopontinhavebeenshowntoreactwithmouseosteopontin.Wehavetakenadvan-tageofthelackofosteopontinproteininmicewithatargeteddisruptionoftheosteopontingenetocharac-terizethereactivityandthespecificityofseveraloftheseantibodieswithmouseosteopontinbyWesternblotting.Ourresultsdemonstratethat,withtheexcep-tionoftheratmonoclonalantibodyMPIIIB10,whichdoesnotrecognizemouseosteopontinonWesternblots,allthetestedreagentsdoreactwithmouseos-teopontin,buttheirsensitivityvarieswidely,andinsomecasesthereissignificantcross-reactivityoftheantibodieswithotherproteinsfoundinmousetissueextracts.©1998AcademicPressThesecretedphosphoproteinosteopontin(OPN)isexpressedinawidevarietyoftissuesinmammals,asassessedbyinsituhybridizationandanalysisofmRNAlevels(1,2).Asasecretedmolecule,however,thelocationoftheproteinitselfmaynotalwayscoin-cidewiththesiteofsynthesis.Immunohistochemicalexperimentshavedemonstratedthepresenceoftheproteininavarietyoftissues,whichinadditiontobone(3,4),mostnotablyincludethosewithsubstantialpop-ulationsofepithelialcellssuchasmammarygland,kidney,andgallbladder(1).Theexpressionoftheproteinisincreasedinavarietyofpathologies,includ-ingtumorigenesis(5–7),avarietyofrenalsyndromes(8–10)andinnumerousformsoftissueinjury(11–14).ThehighlevelofexpressionofOPNinthesesituationsimpliesthattheproteinisplayinganimportantrole,butitsexactfunctionremainsunclear.Wehavedevel-opedastrainofmicewithatargeteddisruptionoftheOPNgene(15).Theseanimalscannotproducefulllengthosteopontinprotein,andsoprovideavaluablesystemforrevealingthefunctionofOPNinnormalandabnormalphysiology.ToassessthelevelofOPNpresentatsteadystateinmousetissues,wehaveusedWesternblottingtodetectOPNintissueextracts.MurineOPNhasapredictedmolecularweightof35,00...