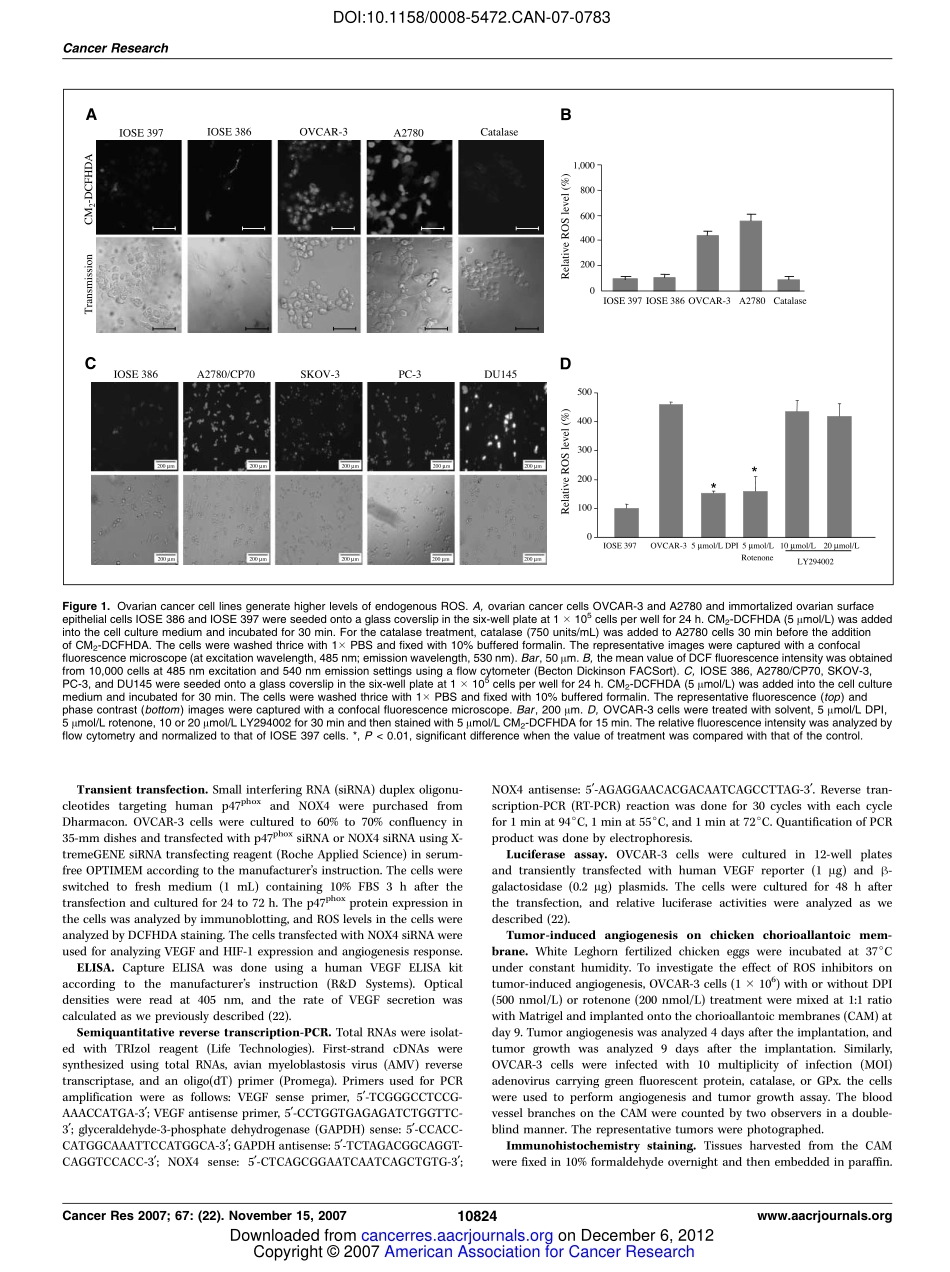
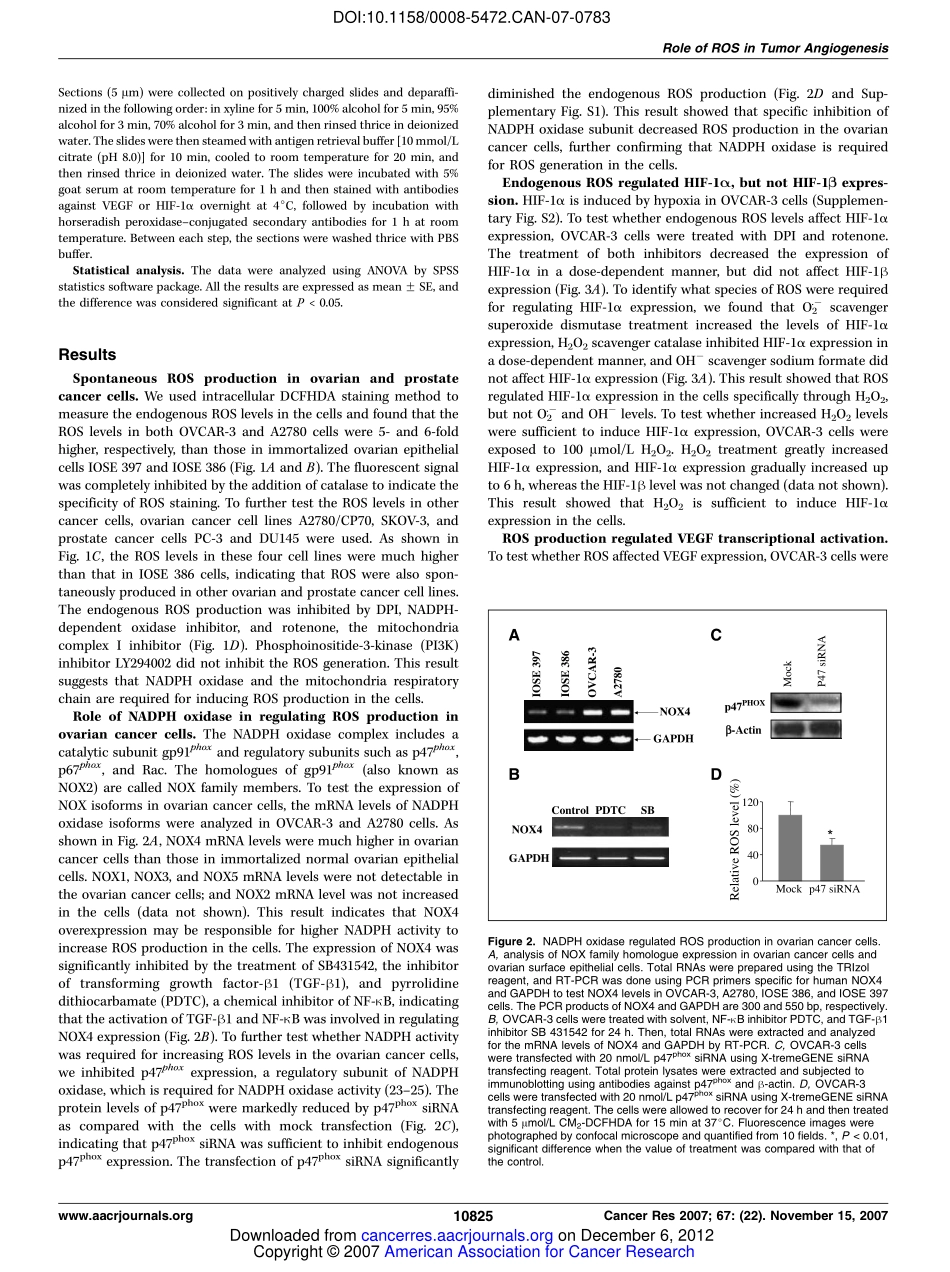
ReactiveOxygenSpeciesRegulateAngiogenesisandTumorGrowththroughVascularEndothelialGrowthFactorChangXia,1QiaoMeng,1Ling-ZhiLiu,1YongyutRojanasakul,2Xin-RuWang,3andBing-HuaJiang11MaryBabbRandolphCancerCenter,andDepartmentsofMicrobiology,ImmunologyandCellBiologyand2PharmaceuticalSciences,WestVirginiaUniversity,Morgantown,WestVirginia;and3LabofReproductiveMedicine,CancerCenter,NanjingMedicalUniversity,Nanjing,Jiangsu,ChinaAbstractReactiveoxygenspecies(ROS)areassociatedwithmultiplecellularfunctionssuchascellproliferation,differentiation,andapoptosis.However,thedirectrolesofendogenousROSproductionstillremaintobeelucidated.Inthisstudy,wefoundthathighlevelsofROSwerespontaneouslyproducedbyovarianandprostatecancercells.ThiselevatedROSproduc-tionwasinhibitedbyNADPHoxidaseinhibitordiphenyleneiodonium(DPI)andmitochondriaelectronchaininhibitorrotenoneinthecells.TofurtheranalyzethesourceofROSproduction,wefoundthatovariancancercellshavemuchhigherexpressionofNOX4NADPHoxidase,andthatspecificinhibitionofNADPHoxidasesubunitp47phoxdiminishedROSproduction.ToanalyzethefunctionalrelevanceofROSproduction,weshowedthatROSregulatedhypoxia-induciblefactor1(HIF-1)andvascularendothelialgrowthfactor(VEGF)expressioninovariancancercells.ElevatedlevelsofendogenousROSwererequiredforinducingangiogenesisandtumorgrowth.NOX4knockdowninovariancancercellsdecreasedthelevelsofVEGFandHIF-1Aandtumorangiogenesis.ThisstudysuggestsanewmechanismofhigherROSproductioninovariancancercellsandprovidesstrongevidencethatendogenousROSplayanimportantroleforcancercellstoinduceangiogenesisandtumorgrowth.ThisinformationmaybeusefultounderstandthenewmechanismofcancercellsininducingtumorigenesisandtodevelopnewtherapeuticstrategybytargetingROSsignalinginhumancancerinthefuture.[CancerRes2007;67(22):10823–30]IntroductionReactiveoxygenspecies(ROS)arenaturallyproducedbycellsthroughaerobicmetabolism,andhighlevelsofROSinthecellsareassociatedwithmanydiseasesincludingcancer(1,2).SeverallinesofevidenceindicatethatROSmaybeinvolvedinhuman...