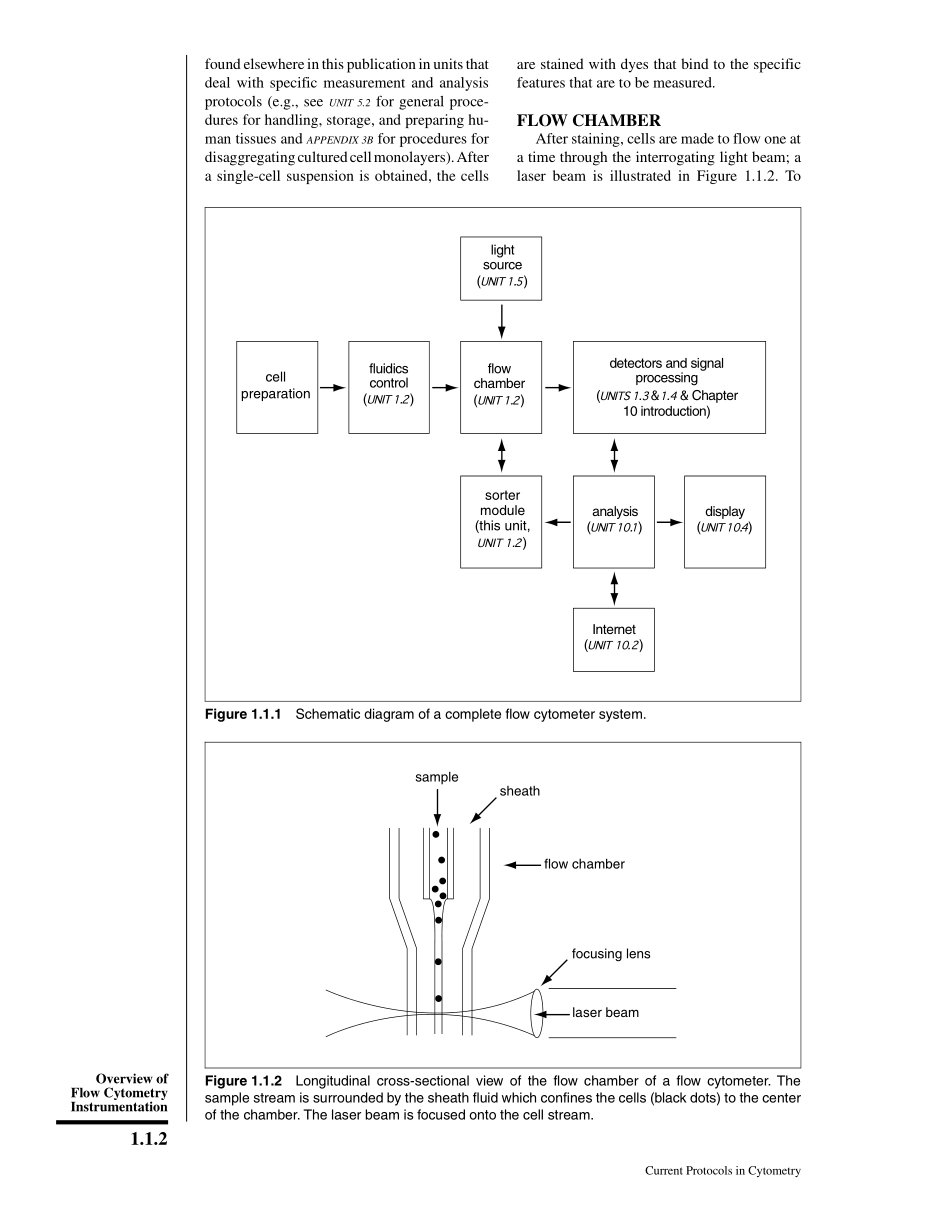
UNIT1.1OverviewofFlowCytometryInstrumentationFlowcytometryisatechnologyinwhichavarietyofmeasurementsaremadeoncells,cellorganelles,andotherobjectssuspendedinaliquidandflowingatratesofseveralthousandpersecondthroughaflowchamber.Flowsort-ingisanextensionofthistechnologyinwhichanysinglecellorobjectmeasuredcanbeselec-tivelyremovedfromthesuspensionbasedonthemeasurementsmade.Flowcytometryisaverybroadlyapplicablemethodology.Abrieflistofapplicationsthatuseflowcytometersincludes:DiseasediagnosisChromosomekaryotypingCellfunctionanalysisCancertherapymonitoringDetectingfetalcellsCellkineticsIdentifyingtumorcellsCytogeneticsFundamentalcellbiology.Inaflowcytometer,cellsinsuspensionaremadetoflowoneatatimethroughasensingregionofaflowchamber(flowcell)wheremeasurementsaremade.AnexampleofanearlyflowcytometeristheCoultercounter(APPENDIX3A).Inthisdevice,cellspassthroughasmallorificeacrosswhichanelectriccurrentisflowing.Asacellenterstheorifice,theflowofcurrentisreducedbecausethecellsarelargelynonconducting.Electroniccircuitsde-tectthedecreaseincurrentandthusthepres-enceofthecell.Inthiswaythedevicecancountthenumberofcellspersecondpassingthroughtheorifice,andbecausethevolumeflowratecanbemeasuredonecandeterminethenumberofcellspermilliliterofsample.TheCoultercounterhasbeeninusesince1949andisstillamainstayoftheclinicallaboratory.Undertherightconditions(e.g.,sizeandlengthoforifice,currentmagnitude),thereductionincurrentthroughtheorificeisproportionaltothesize(volume)ofthecell,asdemonstratedattheLosAlamosScientificLaboratoryin1962.Inmodernflowcytometers,cellsflowthroughalightbeamratherthanthroughaCoulterorifice;aCoulterorificecan,however,beincludedinthesedevices.Manydifferenttypesofmeasurementscanbemadeonthecells,basedonthesizeandshapeofthelightbeamandonthedyesusedtostaincomponentsofinterest.Thelightbeamcancomefromarclamps(e.g.,mercury),asinearlyflowcytome-ters,orfromlasers.Methodsofmeasurementincludeabsorptionandscatteringofthelightbeambythecell,fluorescenceofattachedfluo-rescentdyes,andshap...