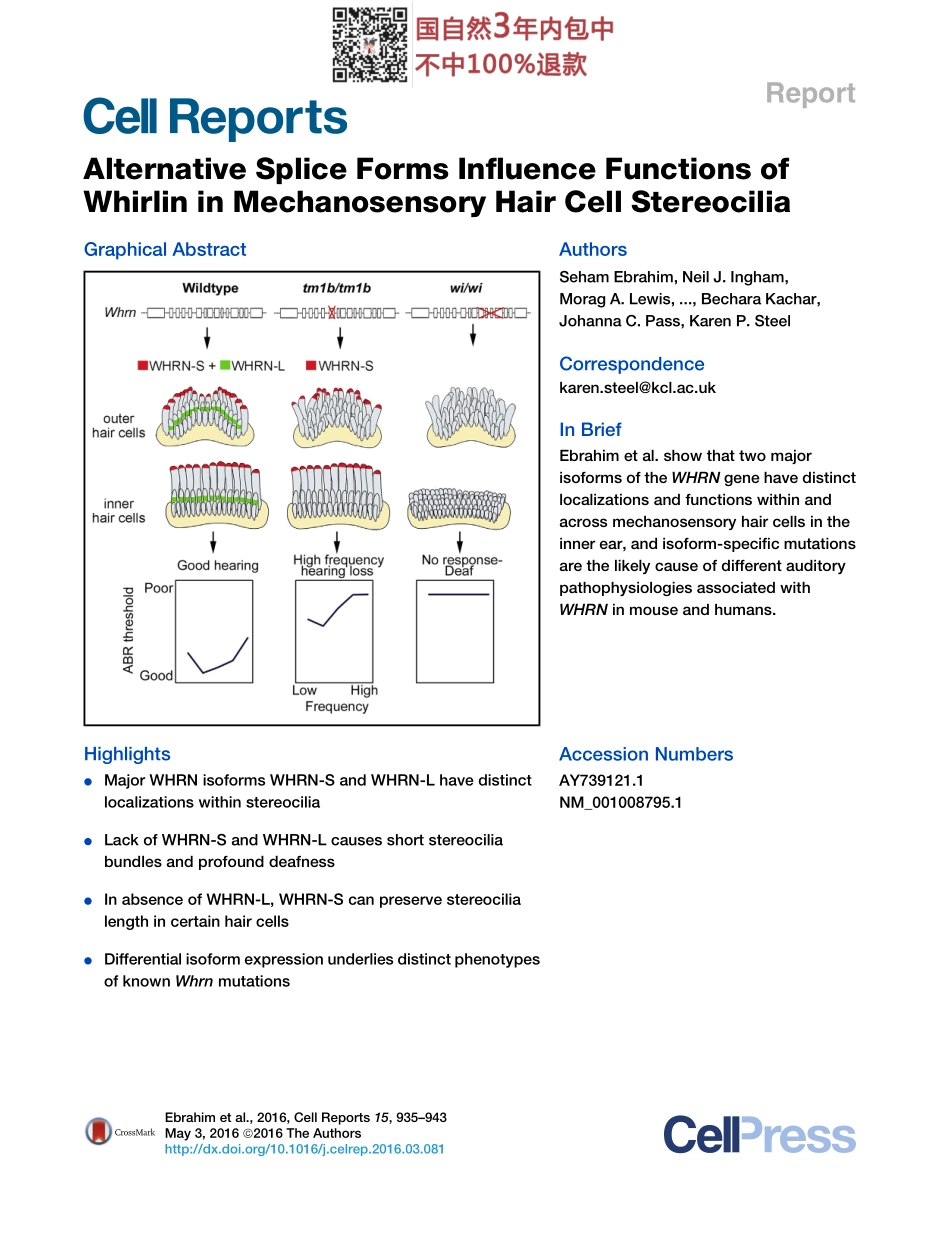
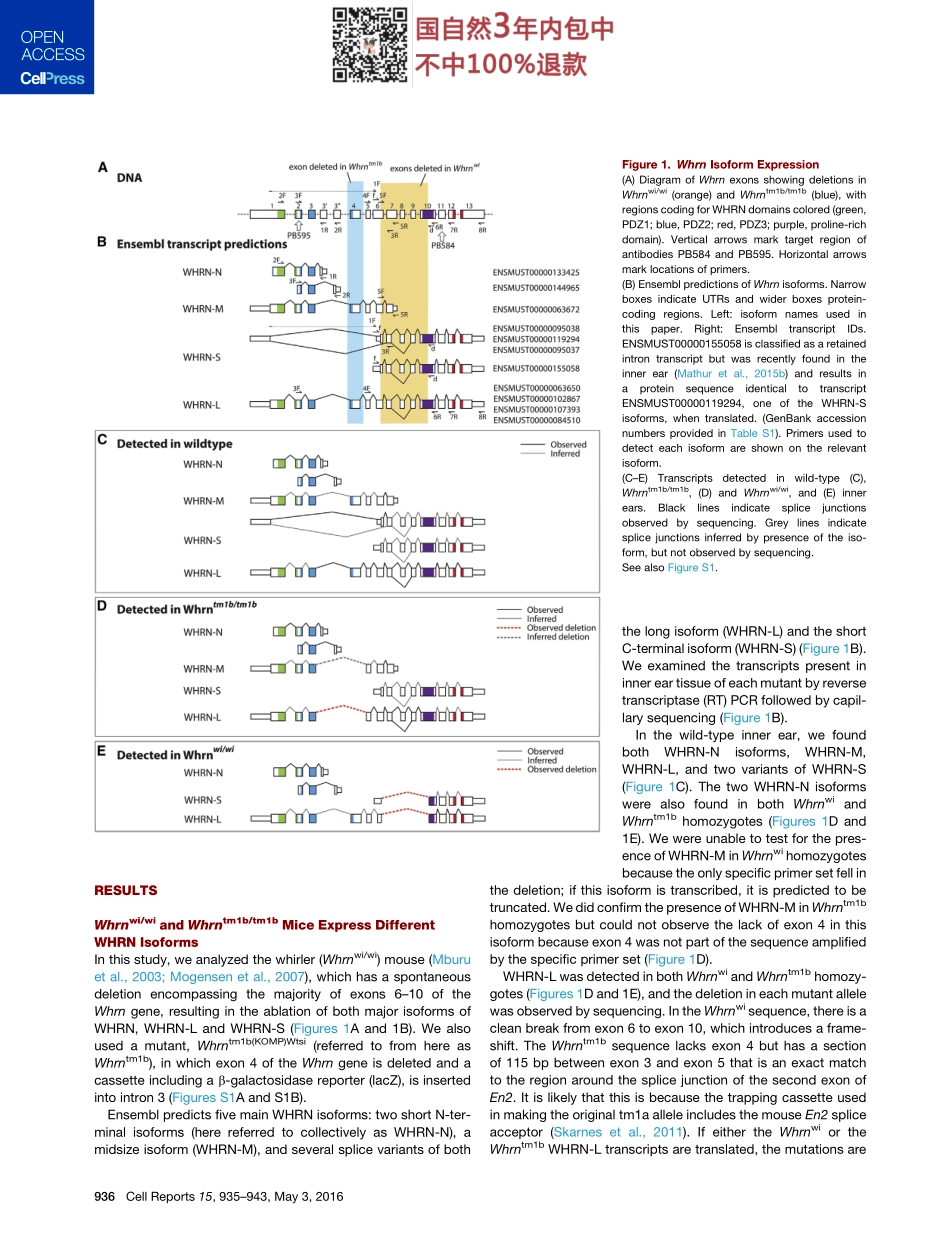
ReportAlternativeSpliceFormsInfluenceFunctionsofWhirlininMechanosensoryHairCellStereociliaGraphicalAbstractHighlightsdMajorWHRNisoformsWHRN-SandWHRN-LhavedistinctlocalizationswithinstereociliadLackofWHRN-SandWHRN-LcausesshortstereociliabundlesandprofounddeafnessdInabsenceofWHRN-L,WHRN-ScanpreservestereocilialengthincertainhaircellsdDifferentialisoformexpressionunderliesdistinctphenotypesofknownWhrnmutationsAuthorsSehamEbrahim,NeilJ.Ingham,MoragA.Lewis,...,BecharaKachar,JohannaC.Pass,KarenP.SteelCorrespondencekaren.steel@kcl.ac.ukInBriefEbrahimetal.showthattwomajorisoformsoftheWHRNgenehavedistinctlocalizationsandfunctionswithinandacrossmechanosensoryhaircellsintheinnerear,andisoform-specificmutationsarethelikelycauseofdifferentauditorypathophysiologiesassociatedwithWHRNinmouseandhumans.AccessionNumbersAY739121.1NM_001008795.1Ebrahimetal.,2016,CellReports15,935–943May3,2016ª2016TheAuthorshttp://dx.doi.org/10.1016/j.celrep.2016.03.081CellReportsReportAlternativeSpliceFormsInfluenceFunctionsofWhirlininMechanosensoryHairCellStereociliaSehamEbrahim,1NeilJ.Ingham,1,2MoragA.Lewis,1MichaelJ.C.Rogers,3RunjiaCui,4BecharaKachar,4JohannaC.Pass,1,2andKarenP.Steel1,2,3,*1WolfsonCentreforAge-RelatedDiseases,King’sCollegeLondon,Guy’sCampus,LondonSE11UL,UK2WellcomeTrustSangerInstitute,Hinxton,CambridgeCB101SA,UK3MRCInstituteofHearingResearch,NottinghamNG72RD,UK4NationalInstituteonDeafnessandOtherCommunicationsDisorders,NIH,Bethesda,MD20892,USA*Correspondence:karen.steel@kcl.ac.ukhttp://dx.doi.org/10.1016/j.celrep.2016.03.081SUMMARYWHRN(DFNB31)mutationscausediversehearingdisorders:profounddeafness(DFNB31)orvariablehearinglossinUshersyndrometypeII.TheknownroleofWHRNinstereociliaelongationdoesnotexplainthesedifferentpathophysiologies.UsingspontaneousandtargetedWhrnmutants,weshowthatthemajorlong(WHRN-L)andshort(WHRN-S)isoformsofWHRNhavedistinctlocalizationswithinstereociliaandalsoacrosshaircelltypes.Lackofbothisoformscausesabnormallyshortstereociliaandprofounddeafnessandvestibular...