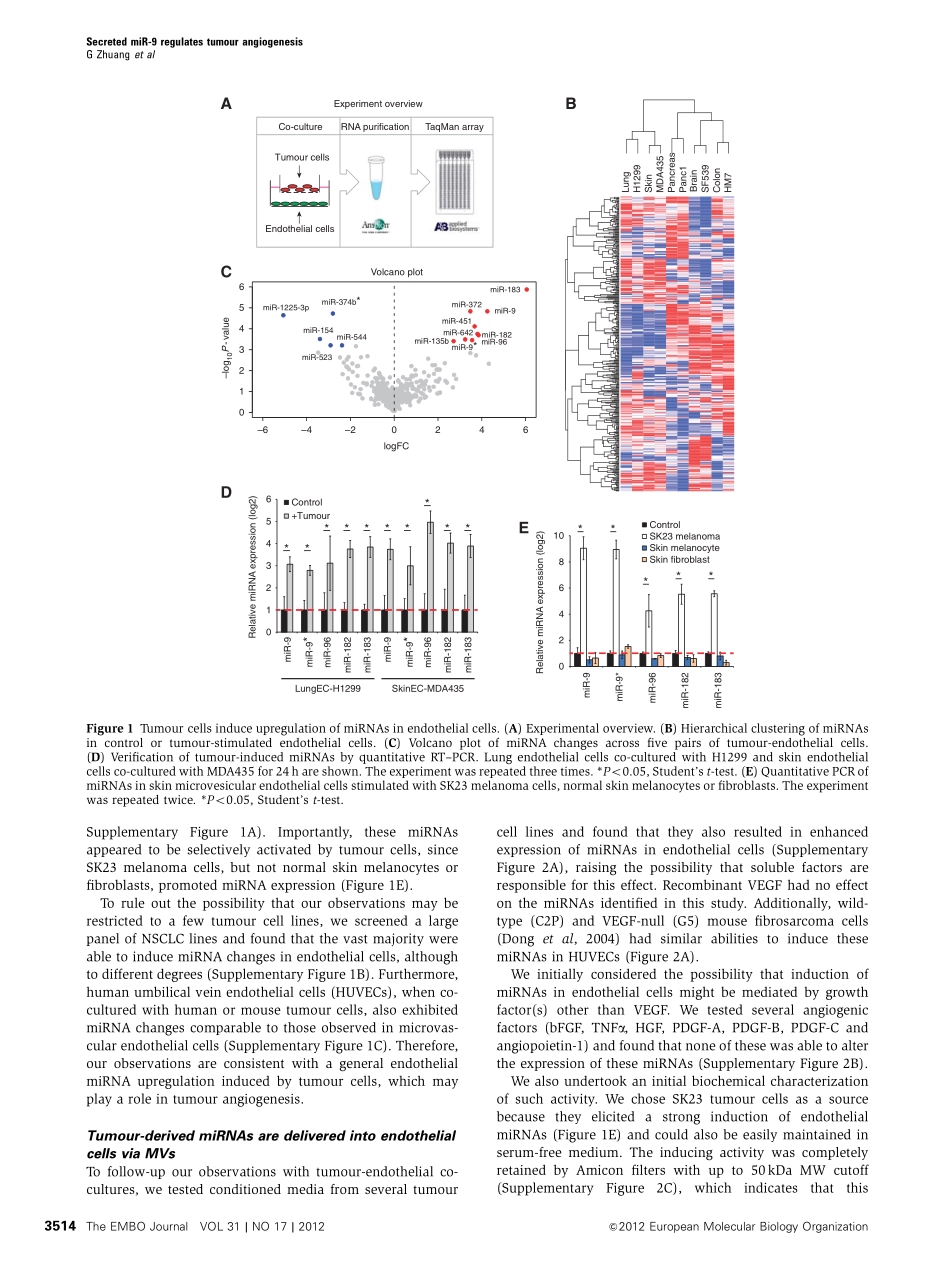
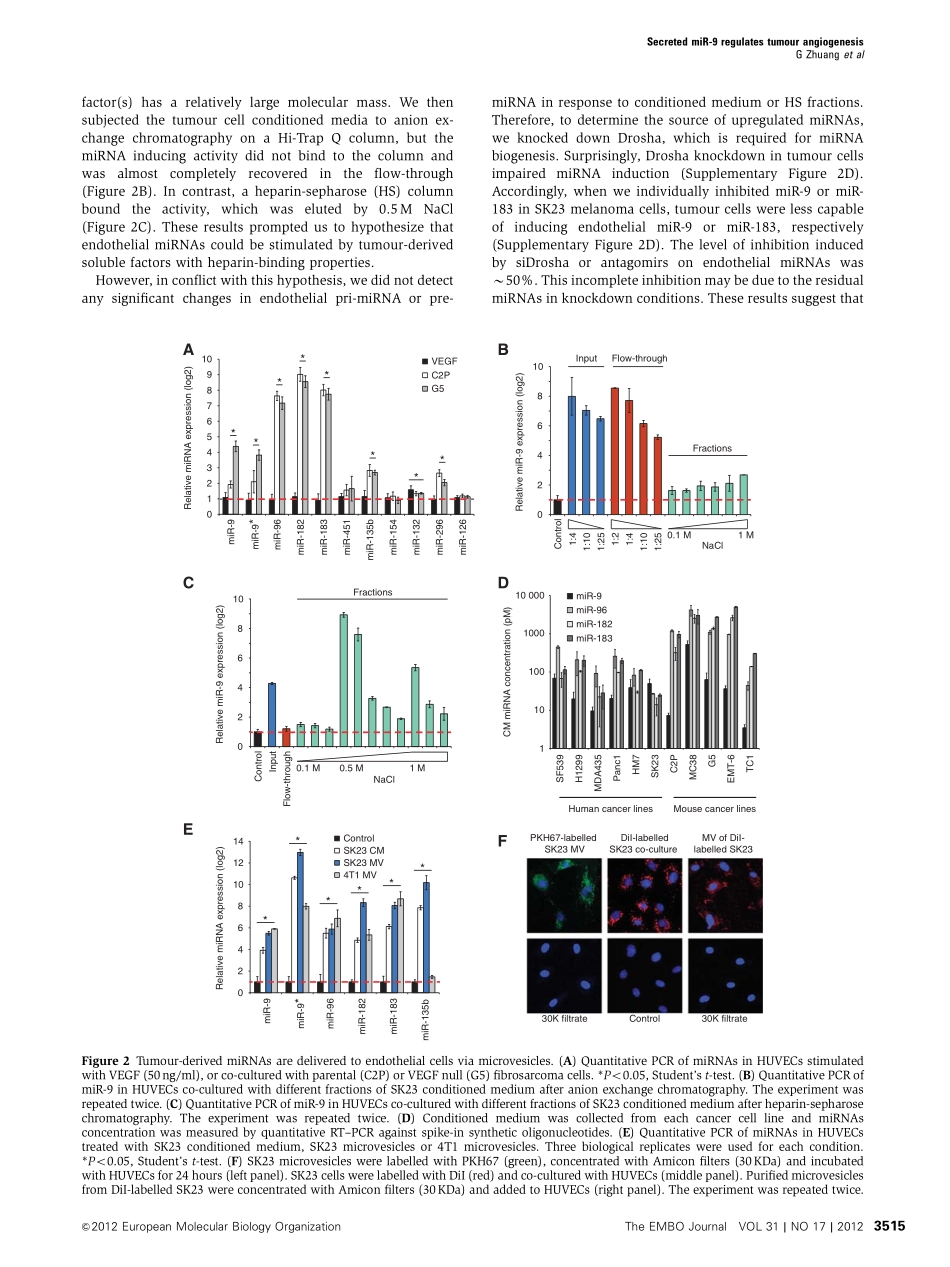
Tumour-secretedmiR-9promotesendothelialcellmigrationandangiogenesisbyactivatingtheJAK-STATpathwayGuangleiZhuang,XiuminWu,ZhaoshiJiang,IanKasman,JennyYao,YinghuiGuan,JasonOeh,ZoraModrusan,CarlosBais,DeepakSampathandNapoleoneFerrara*GenentechInc.,SouthSanFrancisco,CA,USAAngiogenesisplaysacrucialroleduringtumorigenesisandmuchprogresshasbeenrecentlymadeinelucidatingtheroleofVEGFandothergrowthfactorsintheregulationofangiogenesis.Recently,microRNAs(miRNAs)havebeenshowntomodulateavarietyofphysiogicalandpathologicalprocesses.WeidentifiedasetofdifferentiallyexpressedmiRNAsinmicrovascularendothelialcellsco-culturedwithtumourcells.Unexpectedly,mostmiRNAswerede-rivedfromtumourcells,packagedintomicrovesicles(MVs),andthendirectlydeliveredtoendothelialcells.AmongthesemiRNAs,wefocusedonmiR-9duetothestrongmorpholo-gicalchangesinducedinculturedendothelialcells.WefoundthatexogenousmiR-9effectivelyreducedSOCS5levels,leadingtoactivatedJAK-STATpathway.Thissignallingcascadepromotedendothelialcellmigrationandtumourangiogenesis.Remarkably,administrationofanti-miR-9orJAKinhibitorssuppressedMV-inducedcellmigrationinvitroanddecreasedtumourburdeninvivo.Collectively,theseobservationssuggestthattumour-secretedmiRNAsparticipateinintercellularcommunicationandfunctionasanovelpro-angiogenicmechanism.TheEMBOJournal(2012)31,3513–3523.doi:10.1038/emboj.2012.183;Publishedonline6July2012SubjectCategories:RNA;molecularbiologyofdiseaseKeywords:angiogenesis;endothelium;JAK-STAT;microRNA;tumourIntroductionAngiogenesisisakeyrequirementfortumourgrowth(Folkman,1995;Chungetal,2010).MuchevidenceindicatesthatVEGFisamajorregulatorofangiogenesis(ChungandFerrara,2011).VariousinhibitorsoftheVEGFsignallingpathwayhavebeendevelopedandapprovedfortreatmentofcancerorintraocularneovasculardisorders(Ferraraetal,2007;EbosandKerbel,2011).Recently,microRNAs(miRNAs)havebeenshowntoprovideanewlayerofregulationofgeneexpressioninmanyphysiologicalandpathologicalprocesses,includingangiogenesis(WangandOlson,2009;AnandandCheresh,2011;...