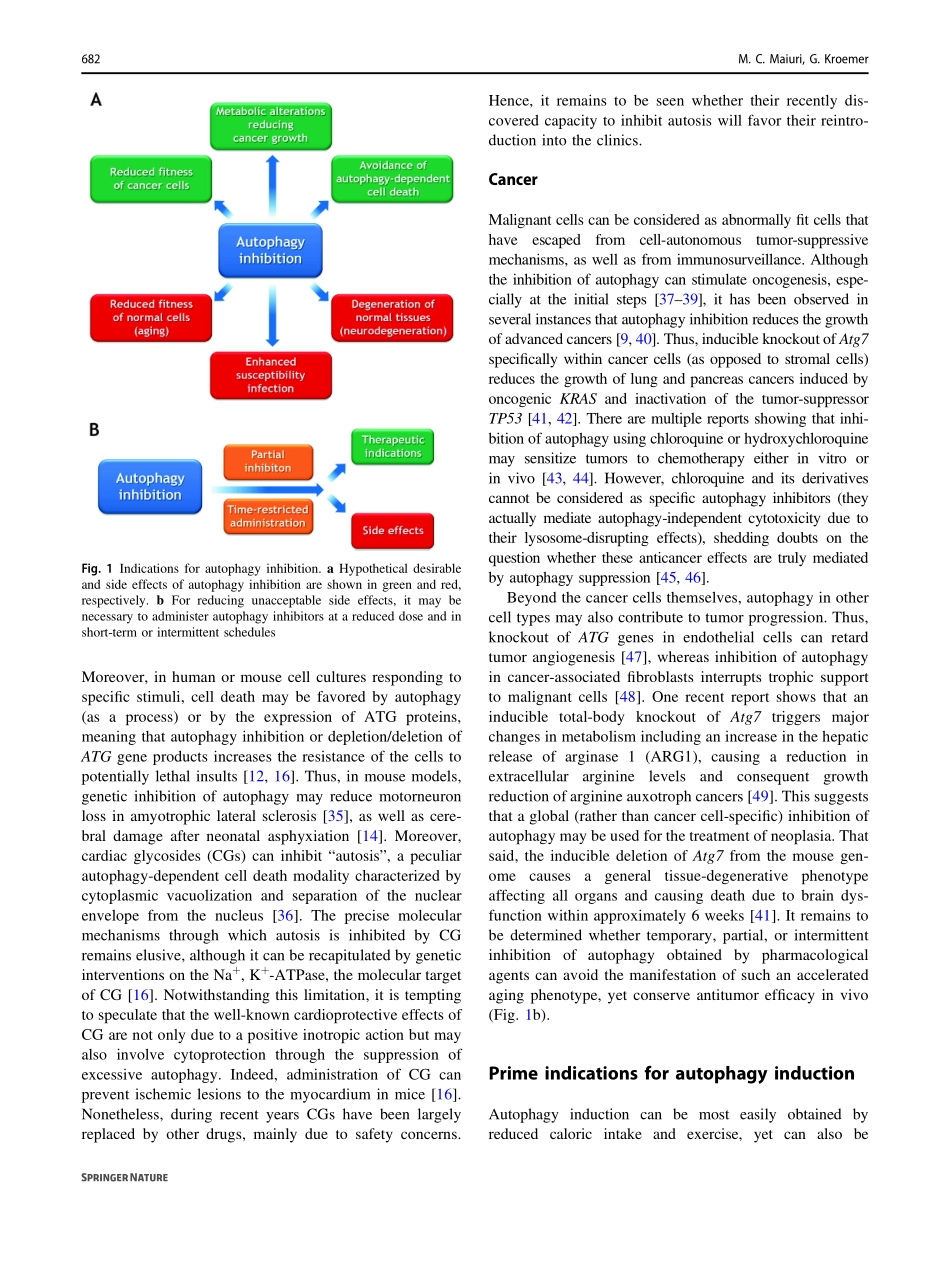
CellDeath&Differentiation(2019)26:680–689https://doi.org/10.1038/s41418-019-0290-0REVIEWARTICLETherapeuticmodulationofautophagy:whichdiseasecomesfirst?MariaChiaraMaiuri1,2,3,4,5●GuidoKroemer2,3,4,5,6,7Received:11January2019/Accepted:11January2019/Publishedonline:6February2019©ADMCAssociazioneDifferenziamentoeMorteCellulare2019AbstractTherelentlesseffortsofthousandsofresearchershavealloweddecipheringthemolecularmachinerythatregulatesandexecutesautophagy,thusidentifyingmultiplemoleculartargetstoenhanceorblocktheprocess,renderingautophagy“druggable”.Autophagyinhibitionmaybeusefulforpreservingthelifeofcellsthatotherwisewouldsuccumbtoexcessiveself-digestion.Moreover,autophagyblockademayreducethefitnessofcancercellsorinterruptmetaboliccircuitriesrequiredfortheirgrowth.Autophagystimulationisprobablyusefulforthepreventionortreatmentofaging,cancer(whenstimulationofimmunosurveillanceisthetherapeuticgoal),cardiovasculardisease,cysticfibrosis,infectionbyintracellularpathogens,obesity,andintoxicationbyheavymetals,justtomentionafewexamples.Epidemiologicalevidencesuggestsbroadhealth-improvingeffectsforlifestyles,micronutrients,anddrugsthatfavorautophagy.Inthisreview,wediscusstheroleofautophagyindiseasepathogenesiswhilefocusingonthequestion,whichdiseasewillbecomethefirstclinicallyapprovedindicationfortherapeuticautophagymodulation.Facts●Autophagyisoneofthebest-studiedphenomenaincellbiology.Drugsforenhancingorinhibitinggeneralorspecificautophagyarebeingdeveloped.●Acquiredorgeneticallydeterminedalterationsinautophagicfluxareinvolvedinmultiplepathologiesacrosstheentirespectrumofhumandiseases.●Autophagyinhibitionmightbeusefulfortheavoidanceofunwarrantedautophagy-dependentcelldeath.●Chronicautophagystimulationhasapositiveimpactonpreclinicalmodelsofagingandmultipledistinctage-dependentdiseases,includingarteriosclerosis,cancer,andneurodegeneration.Acuteautophagystimulationalsohasorgan-protectiveeffectsinmodelsofischemiaorintoxication.Openquestions●Optimalpharmacologicalagents...