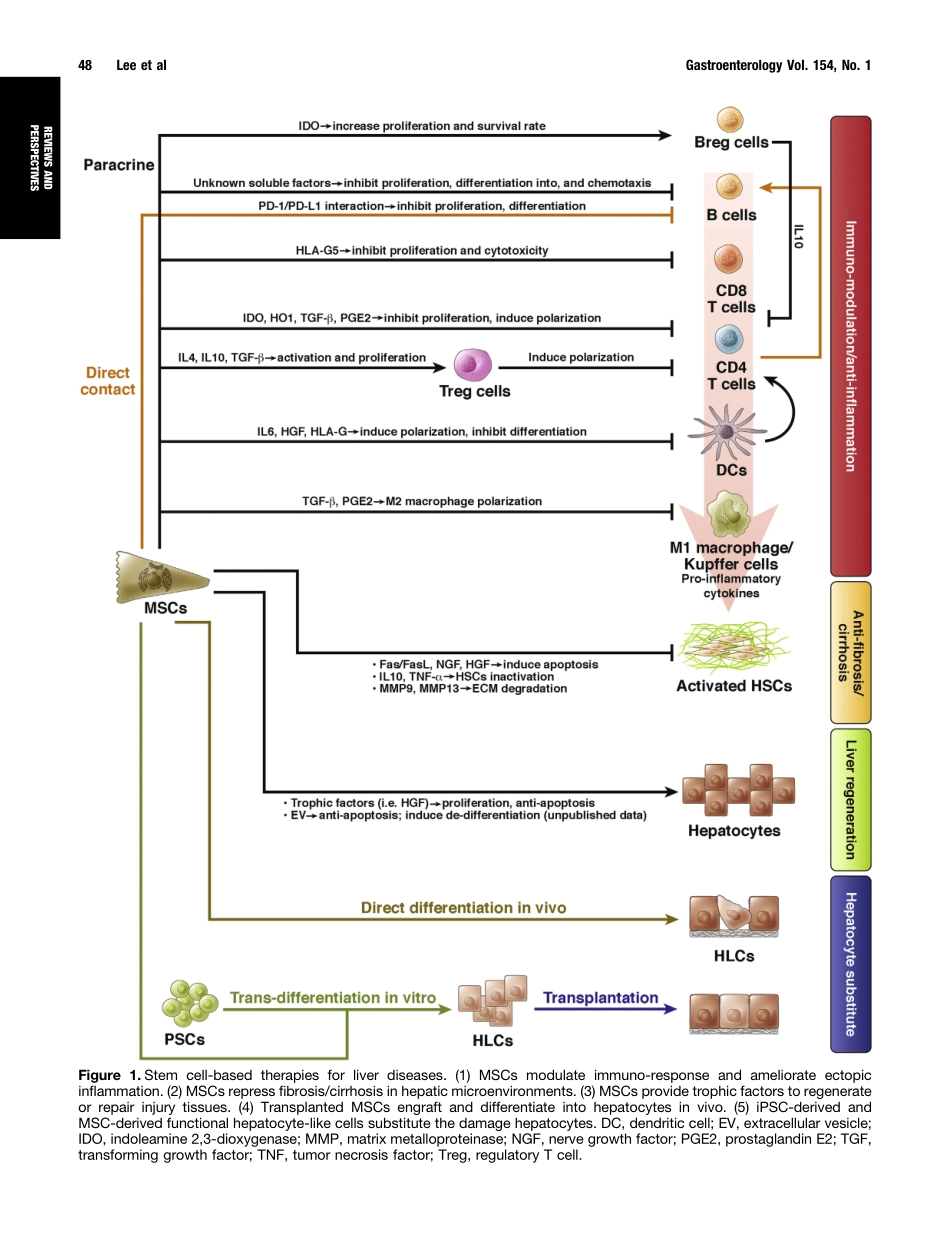
HistoricalPerspectivesandAdvancesinMesenchymalStemCellResearchfortheTreatmentofLiverDiseasesChien-WeiLee,1,2Yu-FanChen,2,3Hao-HsiangWu,2,4andOscarK.Lee2,3,5,61PrograminMolecularMedicine,NationalYang-MingUniversityandAcademiaSinica,Taipei,Taiwan;2StemCellResearchCenter,NationalYang-MingUniversity,Taipei,Taiwan;3InstituteofClinicalMedicine,NationalYang-MingUniversity,Taipei,Taiwan;4InstituteofBiophotonics,NationalYang-MingUniversity,Taipei,Taiwan;5DepartmentofMedicalResearch,TaipeiVeteransGeneralHospital,Taipei,Taiwan;and6TaipeiCityHospital,Taipei,TaiwanLivertransplantationistheonlyeffectivetherapyforpatientswithdecompensatedcirrhosisandfulminantliverfailure.However,duetoashortageofdonorliversandcomplicationsassociatedwithimmunesuppression,thereisanurgentneedfornewtherapeuticstrategiesforpatientswithend-stageliverdiseases.Giventheiruniquefunctioninself-renewalanddifferentiationpotential,stemcellsmightbeusedtoregeneratedamagedlivertissue.RecentstudieshaveshownthatstemcellLbasedtherapiescanimproveliverfunctioninamousemodelofhepaticfailure.Moreover,acellularliverscaffoldsseededwithhepatocytesproducedfunctionalbioengineeredliversfororgantransplantationinpreclinicalstudies.Thethera-peuticpotentialofstemcellsortheirdifferentiatedprog-enieswilldependontheircapacitytodifferentiateintomatureandfunctionalcelltypesaftertransplantation.Itwillalsobeimportanttodevisemethodstoovercometheirgenomicinstability,immunereactivity,andtumorigenicpotential.Wereviewdirectionsandadvancesintheuseofmesenchymalstemcellsandtheirderivedhepatocytesforliverregeneration.Wealsodiscussthepotentialapplicationsofhepatocytesderivedfromhumanpluripo-tentstemcellsandchallengestousingthesecellsintreatingend-stageliverdisease.Keywords:StemCellTherapy;PluripotentStemCells;Hepatocytes;RegenerativeMedicine.Despitethesuccessoftransplantationofhematopoieticstemcells,fewothercell-basedtherapieshavebeensuccessfullytranslatedintoclinicalapplication.Thedearthofcell-basedtherapiesisunfortu-nate,asthepotentialanddemand...