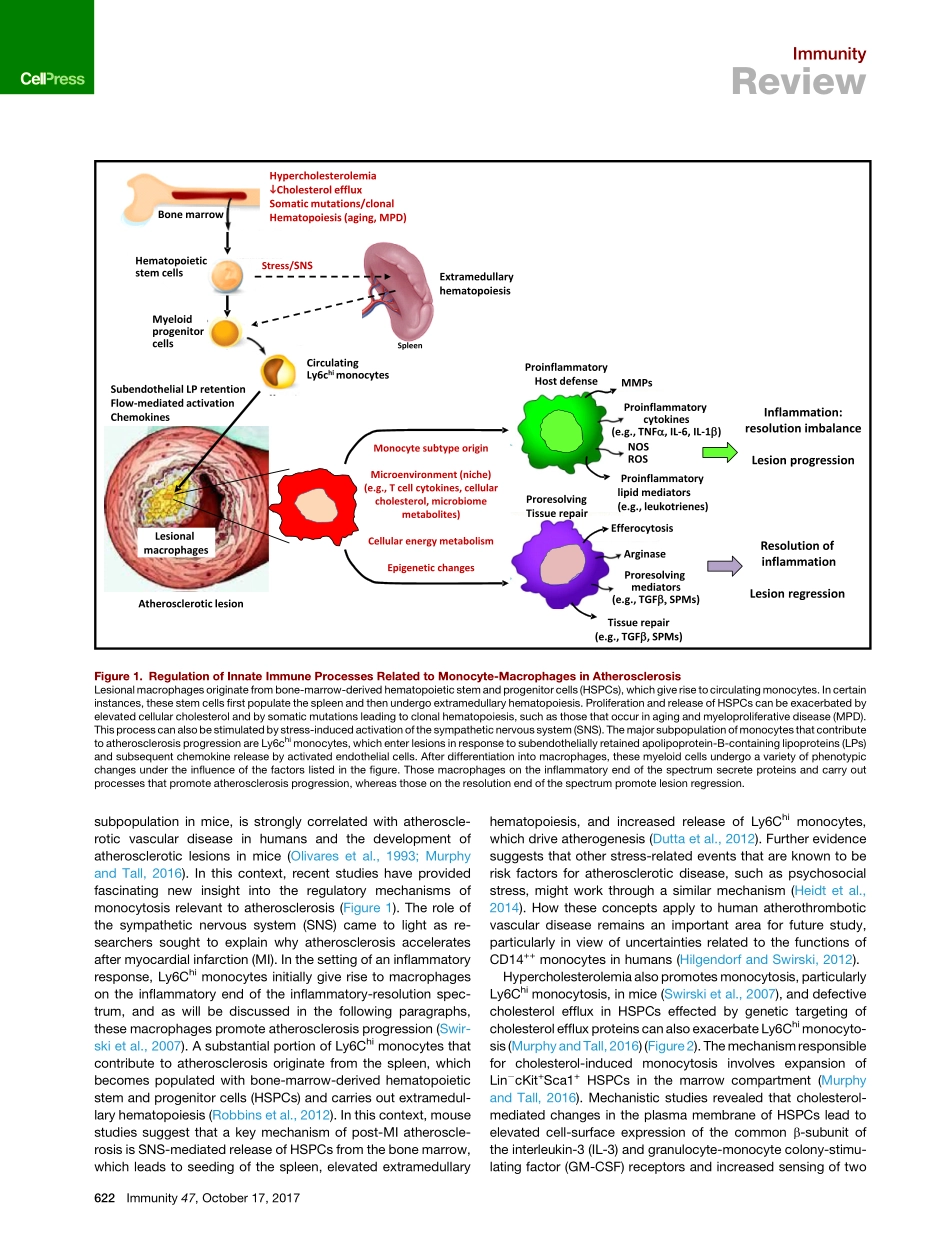
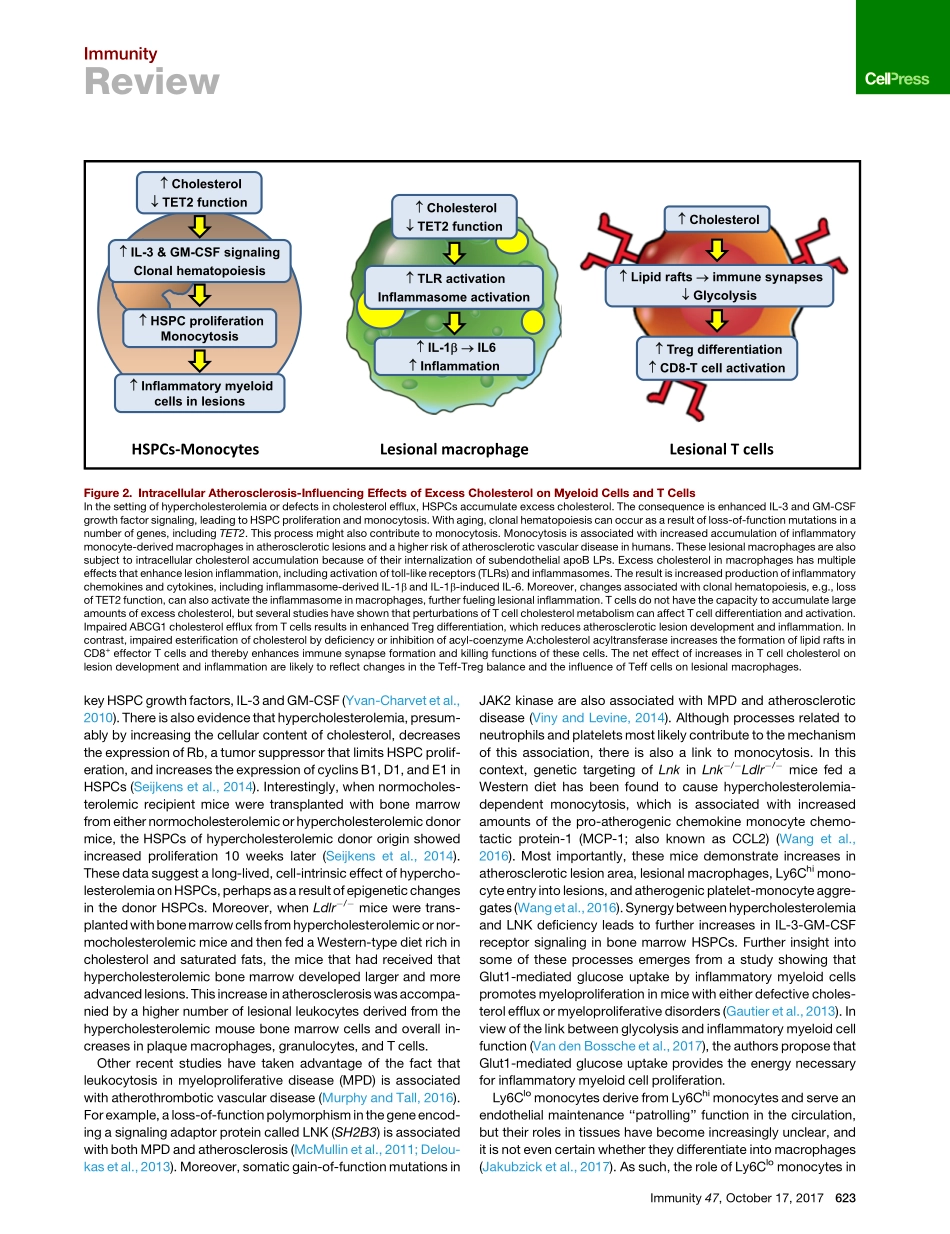
ImmunityReviewMonocyte-MacrophagesandTCellsinAtherosclerosisIraTabas1,*andAndrewH.Lichtman2,*1DepartmentsofMedicine,Physiology,andPathology&CellBiology,ColumbiaUniversityMedicalCenter,NewYork,NY10032,USA2DepartmentofPathology,BrighamandWomen’sHospitalandHarvardMedicalSchool,Boston,MA02115,USA*Correspondence:iat1@columbia.edu(I.T.),alichtman@bwh.harvard.edu(A.H.L.)https://doi.org/10.1016/j.immuni.2017.09.008Atherosclerosisisanarterialdiseaseprocesscharacterizedbythefocalsubendothelialaccumulationofapolipoprotein-B-containinglipoproteins,immuneandvascularwallcells,andextracellularmatrix.Thelipoproteinsacquirefeaturesofdamage-associatedmolecularpatternsandtriggerfirstaninnateimmuneresponse,dominatedbymonocyte-macrophages,andthenanadaptiveimmuneresponse.Theseinflamma-toryresponsesoftenbecomechronicandnon-resolvingandcanleadtoarterialdamageandthrombosis-inducedorganinfarction.Theinnateimmuneresponseisregulatedatvariousstages,fromhematopoiesistomonocytechangesandmacrophageactivation.TheadaptiveimmuneresponseisregulatedprimarilybymechanismsthataffectthebalancebetweenregulatoryandeffectorTcells.Mechanismsrelatedtocellularcholesterol,phenotypicplasticity,metabolism,andagingplaykeyrolesinaffectingtheseresponses.Herein,wereviewselecttopicsthatshedlightontheseprocessesandsuggestnewtreatmentstrategies.ABriefOverviewofAtherogenesisandAtheroscleroticPlaqueProgressionAtherogenesisisinitiatedbytheentryandretentionofapolipo-protein-B-containinglipoproteins(apoBLPs)intothesubendo-thelialspace,or‘‘intima,’’atregionsofdisturbedbloodflowinmedium-sizedarteries(WilliamsandTabas,1995;FogelstrandandBore´n,2012).TheamountofapoBLPretentionisdeter-minedbytheconcentrationofapoBLPsintheblood,theageandmetabolicstateoftheindividual,andgeneticandenviron-mentalfactors.Theseconsiderationsaffectarterialwallbiology,includingvariationsinsubendothelialproteoglycansthatretainapoBLPsandfactorsthatalterendothelialpermeability.Initially,someofthelipoproteinlipidisinternalizedbyresidentCD11c+myeloidce...