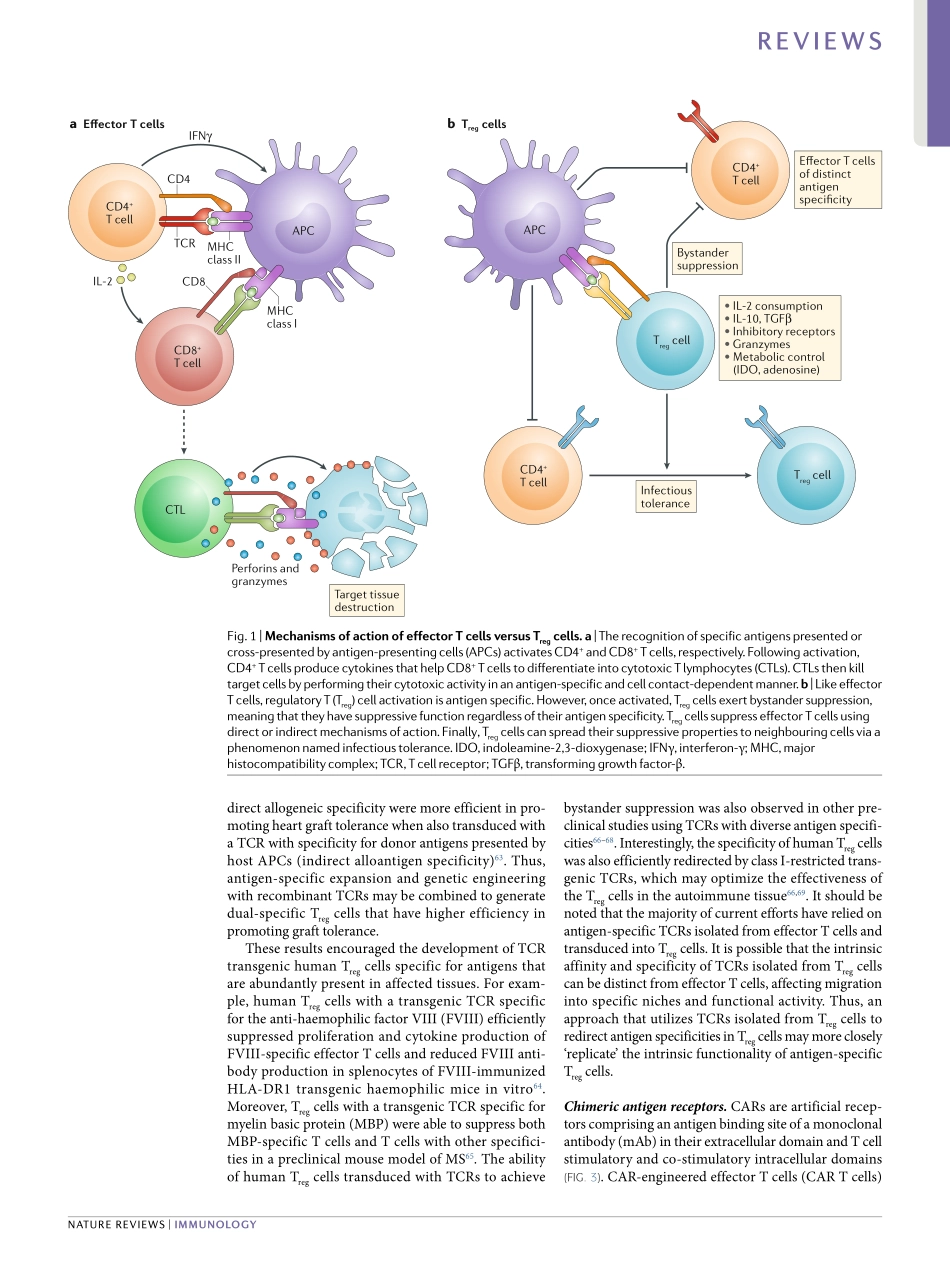
ThetherapeuticpotentialofauniqueFOXP3+immunosuppressivesubsetofregulatoryT(Treg)cellshasbeendemonstratedinvariouspreclinicalmodelsofgraftversushostdisease(GVHD)1–3,solidorgantransplantation4,5,type1diabetesmellitus(T1D)6,7,systemiclupuserythematosus(SLE)8,inflammatoryboweldisease9,10andmultiplesclerosis(MS)11.Thishasstimulatedadvancesintheclinicaldevelopmentofadoptivecelltherapy(ACT)ofTregcellsintheclinic.Therearenowmorethan50activeandcompletedclinicaltrialstestingthesafetyandefficacyofTregcelltherapyforindicationssuchaskidneyorlivertransplantation,pemphigusvulgaris,SLE,inflammatoryboweldisease,autoimmunehepatitis,allergyandasthma12.AlthoughseparationandexpansionprotocolsfortheTregcellsvary,earlyclinicaltrialresultsreportmanufacturingsuccessandexcellentsafetyprofiles12–17.BroadeningthetherapeuticpotentialofTregcellACTwilldependontheuseofnoveltechnologiestoalterthegenomeofthecellstoenhancefunctionalactivity,stability,persistenceandantigenspecificity.NumerouspreclinicalstudieshavedemonstratedthatantigenspecificTregcellsaremorepotentthanpolyclonalTregcellsinmodelsofT1D6,7,autoimmunecentralnervoussystemdisease18andtransplantation19–22.Moreover,antigenspecificTregcellspredominantlylocalizeatthesiteofantigenpresentation,decreasingtheriskofgeneralizedimmunosuppression.Thus,antigenspecificTregcellsmaybebothsaferandmoreefficientthanunselectedpolyclonalTregcellsforACT.Becauseoftheirhighprecursorfrequency23,24,‘antigenselected’humanTregcellswithdirectallospecificity,derivedfromthetransplantrecipient,canbeefficientlyexpandedinvitrousingallogeneicantigenpresentingcells(APCs)fromthetransplantdonor25.Thisiscurrentlybeingevaluatedasacellbasedtherapyinseveralclinicaltrials26,27.However,TregcellswithindirectallospecificityaswellasselfantigenreactiveTregcellshaveaprecursorfrequencypredictedtobe1,000foldto10,000foldlowerthanthefrequencyofdirectalloreactiveTregcellsandhavenotbeeneffectivelyexpandedtodate23,28.Hence,alternativestrategiessuc...