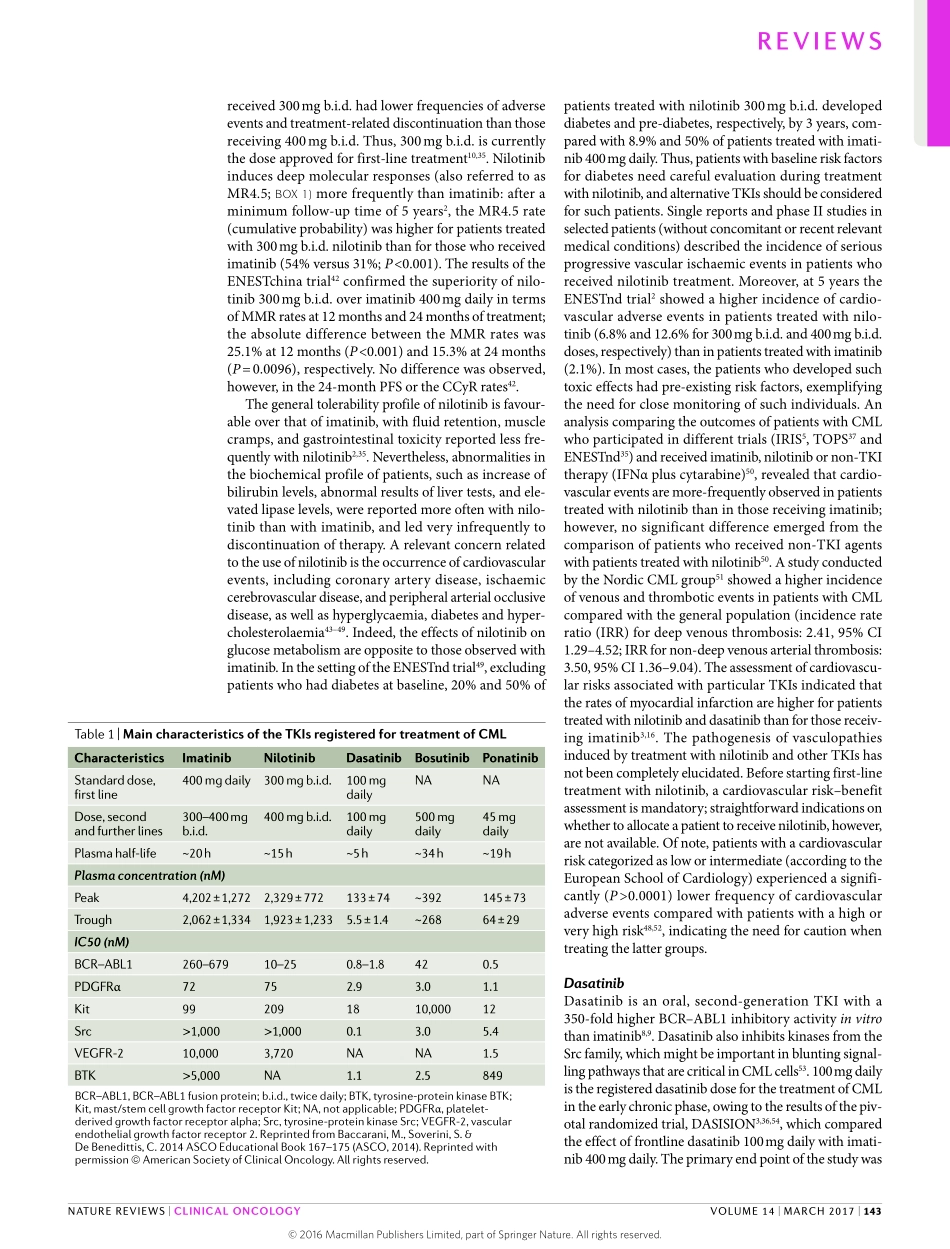
Currently,thetyrosinekinaseinhibitor(TKI)imatinibisthe‘goldstandard’therapyforthetreatmentofchronicmyeloidleukaemia(CML).ImatinibwasthefirstTKIthatwasapprovedinCML,anddramaticallyincreasedtheoverallsurvivalofpatientswithCML,tolevelsnotyetimproveduponbythefirst-linetreatmentwithsecond-generationTKIs1–6.Around30%ofthepatients,however,developresistancetotreatmentwiththestand-arddoseofimatinib(400mgdaily).Inthepastdecade,strategiestoimprovetherapyforsuchpatientswerebasedpredominantlyontheoptimizationofimatinibadministration(forexample,increasingthedose).Thedevelopmentandapprovalofsecond-generationandthird-generationTKIs(dasatinib,nilotinib,bosutinib,andponatinib)ledtoawideravailabilityoftherapeuticagentsforsecond-lineandthird-linetherapy7–10.Atpres-ent,inmanycountries,imatinib,nilotinibanddasatinibareregisteredforfirst-linetreatment,whereasponati-nibandbosutinibareapprovedonlyasrescuestrategies.Approximately,30–50%ofthepatientswhoreceiveimatinibasfrontlinetreatmentwillremainonlong-term(≥5years)treatmentwiththisagent;conversely,alargeproportionofpatients(50–70%)willrequiretreatmentwithasecond-lineorthird-lineagent6–10.Thus,long-term(≥5years)survivaldoesnotdependexclusivelyonthechoiceoffirst-lineagent,butalsoontheavailabilityandproperimplementationofTKIsbeyondfirst-line.InthisReview,wediscussthedebatableanddifficultchoiceoffirstandsubsequentlinesoftreatmentthatphysiciansfacewhentreatingpatientswithCML.AgentsforfrontlinetreatmentofCMLImatinibImatinibwasthefirstTKItobeapprovedbytheFDAforthetreatmentofpatientswithCML1,5.ImatinibinhibitsthekinaseactivityofthefusionproteinBCR–ABL1—encodedbyagenemutatedinallpatientswithPhiladelphia-chromosome-positive(Ph+)CML1,4–6—throughacompetitivemechanismattheATP-bindingsite.Inaddition,imatinibinhibitstheactivityofothertyrosinekinasesrelevanttolymphocytefunction,suchasPGDFR,andthemast/stemcellgrowthfactorreceptorKit10(TABLE1).TheIRISstudy1,5,6wasthefirstclinicaltrialtoestablishthatpatientswithCMLcanbenefitfromtreatmentwithTK...