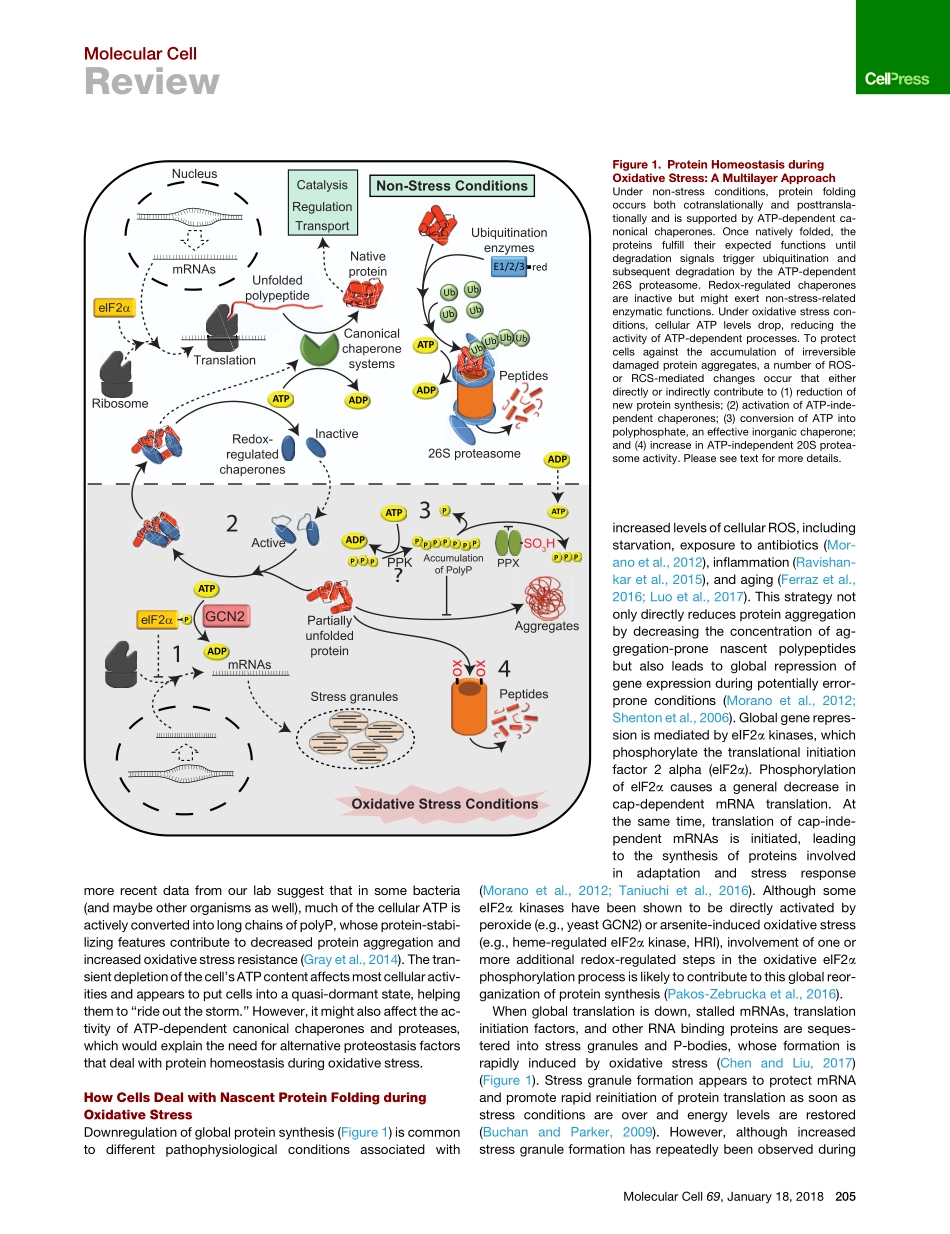
MolecularCellReviewMaintainingaHealthyProteomeduringOxidativeStressDanaReichmann,1,*WilhelmVoth,2andUrsulaJakob2,*1DepartmentofBiologicalChemistry,TheAlexanderSilbermanInstituteofLifeSciences,SafraCampusGivatRam,TheHebrewUniversityofJerusalem,Jerusalem91904,Israel2DepartmentofMolecular,Cellular,andDevelopmentalBiologyandDepartmentofBiologicalChemistry,UniversityofMichigan,AnnArbor,MI48109-1048,USA*Correspondence:danare@mail.huji.ac.il(D.R.),ujakob@umich.edu(U.J.)https://doi.org/10.1016/j.molcel.2017.12.021Someofthemostchallengingstressconditionsthatorganismsencounterduringtheirlifetimeinvolvethetransientaccumulationofreactiveoxygenandchlorinespecies.Extremelyreactivetoaminoacidsidechains,theseoxidantscausewidespreadproteinunfoldingandaggregation.Itisthereforenotsurprisingthatcellsdrawonavarietyofdifferentstrategiestocounteractthedamageandmaintainahealthyproteome.Orchestratedlargelybydirectchangesinthethioloxidationstatusofkeyproteins,theresponsestrategiesinvolvealllayersofproteinprotection.Reprogrammingofbasicbiologicalfunctionshelpsdecreasenascentproteinsynthesisandrestoreredoxhomeostasis.Mobilizationofoxidativestress-activatedchaperonesandproductionofstress-resistantnon-proteinaceouschaperonespreventirreversibleproteinaggregation.Finally,redox-controlledincreaseinproteasomeactivityremovesanyirreversiblydamagedproteins.Together,thesesystemspavethewaytorestoreproteinhomeostasisandenableorganismstosurvivestressconditionsthatareinevitablewhenlivinganaerobiclifestyle.IntroductionProteinsarethemostdiverseandstructurallycomplexmacro-moleculesinthecell.Considerednature’sworkhorses,theyareinvolvedinalmosteveryknownaspectofbiologicalfunction.Thefunctionofproteinsisdefinedbytheirspecificthree-dimen-sionalstructure,makingitatoppriorityforeverycellandorgan-ismtoensurethatnascentpolypeptidechainsadoptandnativeproteinspreservetheirproperlyfoldedconformationevenduringstressfulsituations(Balchinetal.,2016).Controloverahealthyproteome(i.e.,proteostasis)beginswiththebirthofthepo...