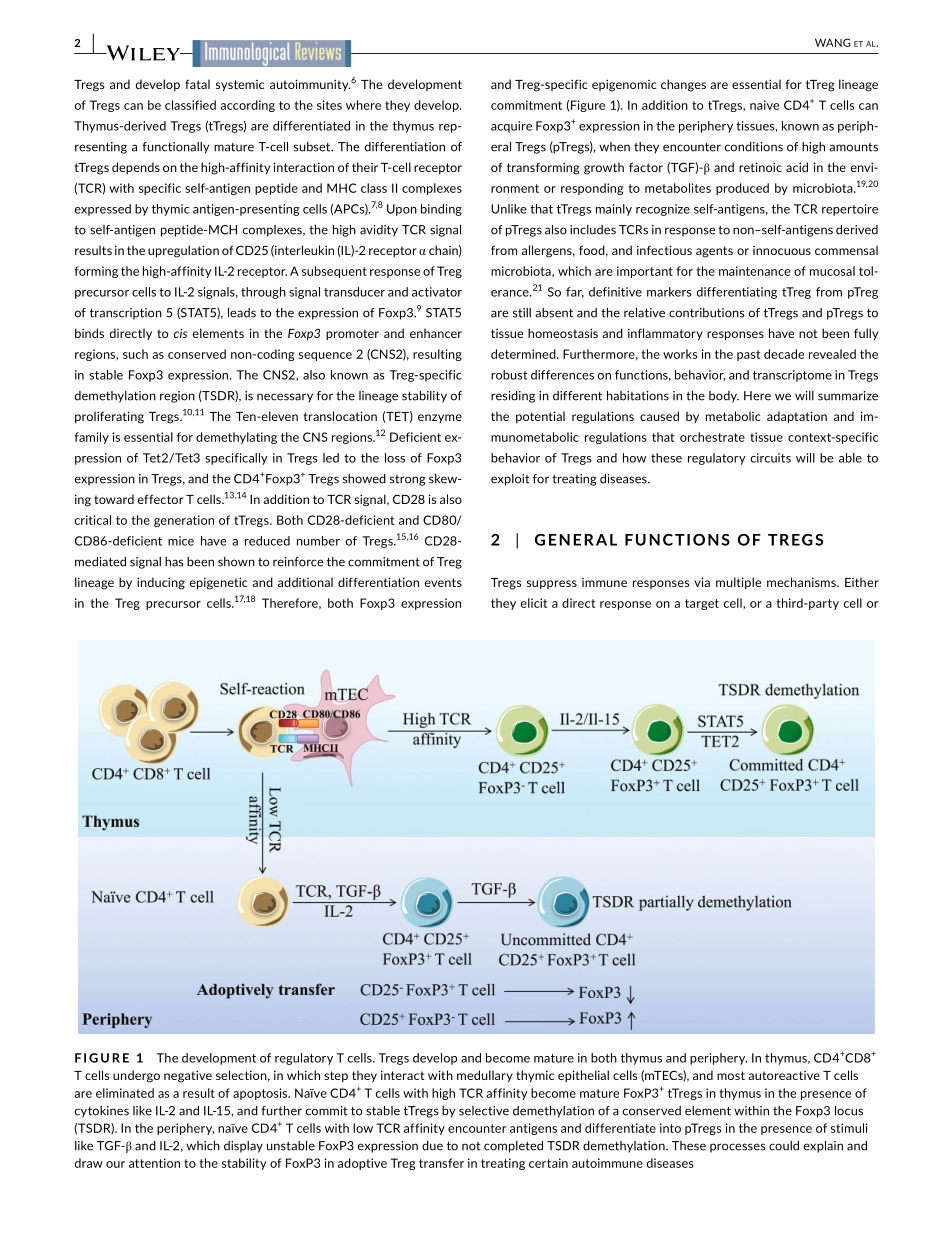
ImmunologicalReviews.2020;00:1–14.|1wileyonlinelibrary.com/journal/imr1|INTRODUCTIONTheimmunesystemismainlyresponsibleforprotectingindividu-alsfrompathogeninvasion.Meanwhile,theimmunesystemalsoactivelyparticipatesintissuerepairingandhomeostasis,whicharecriticalfortheavoidanceofautoimmunity.AsubsetofCD4+Tcells,whichischaracterizedbytheexpressionofthemastertranscriptionfactorforkheadboxproteinP3(Foxp3)andknownasregulatoryTcells(Tregs),isthemajorimmunesubsettomaintaintheimmuneho-meostasis.1-4TheimportanceofTregsongoverningperipheraltissueandimmunehomeostasisisvalidatedbyobservationsinbothhumanpatientsandmurinemodels.Inhumans,themutationsinFoxp3re-sultinimpaireddevelopmentanddysfunctionofTregsandcauseim-munedysregulationpolyendocrinopathyenteropathyX-linked(IPEX)syndrome,characterizedbymultipleautoimmunedisorders.5,6MicecarryingdysfunctionalFoxp3,knownasscurfymice,aredeficientinReceived:29January2020|Accepted:19February2020DOI:10.1111/imr.12844INVITEDREVIEWMetabolicadaptationorchestratestissuecontext-dependentbehaviorinregulatoryTcellsHaipingWang1,2|Chun-HaoLu1,2|Ping-ChihHo1,2©2020JohnWiley&SonsA/S.PublishedbyJohnWiley&SonsLtdThisarticleispartofaseriesofreviewscoveringMetabolicAlterationsinImmuneCellsappearinginVolume295ofImmunologicalReviews.1DepartmentofFundamentalOncology,UniversityofLausanne,Lausanne,Switzerland2LudwigInstituteforCancerResearch,UniversityofLausanne,Epalinges,SwitzerlandCorrespondenceHaipingWang;Ping-ChihHo,DepartmentofFundamentalOncology,LudwigInstituteforCancerResearch,UniversityofLausanne,ChemindesBoveresses155,1066Epalinges,Switzerland.Email:haiping.wang@unil.ch;ping-chih.ho@unil.chFundinginformationH2020EuropeanResearchCouncil,Grant/AwardNumber:ISREC26075483;SwissInstituteforExperimentalCancerResearch,Grant/AwardNumber:ISREC26075483;SNSFprojectgrants,Grant/AwardNumber:31003A_182470AbstractThediversedistributionandfunctionsofregulatoryTcells(Tregs)ensuretissueandimmunehomeostasis;however,itremainsunclearwhichfactorscanguidedistribu-tion,loc...