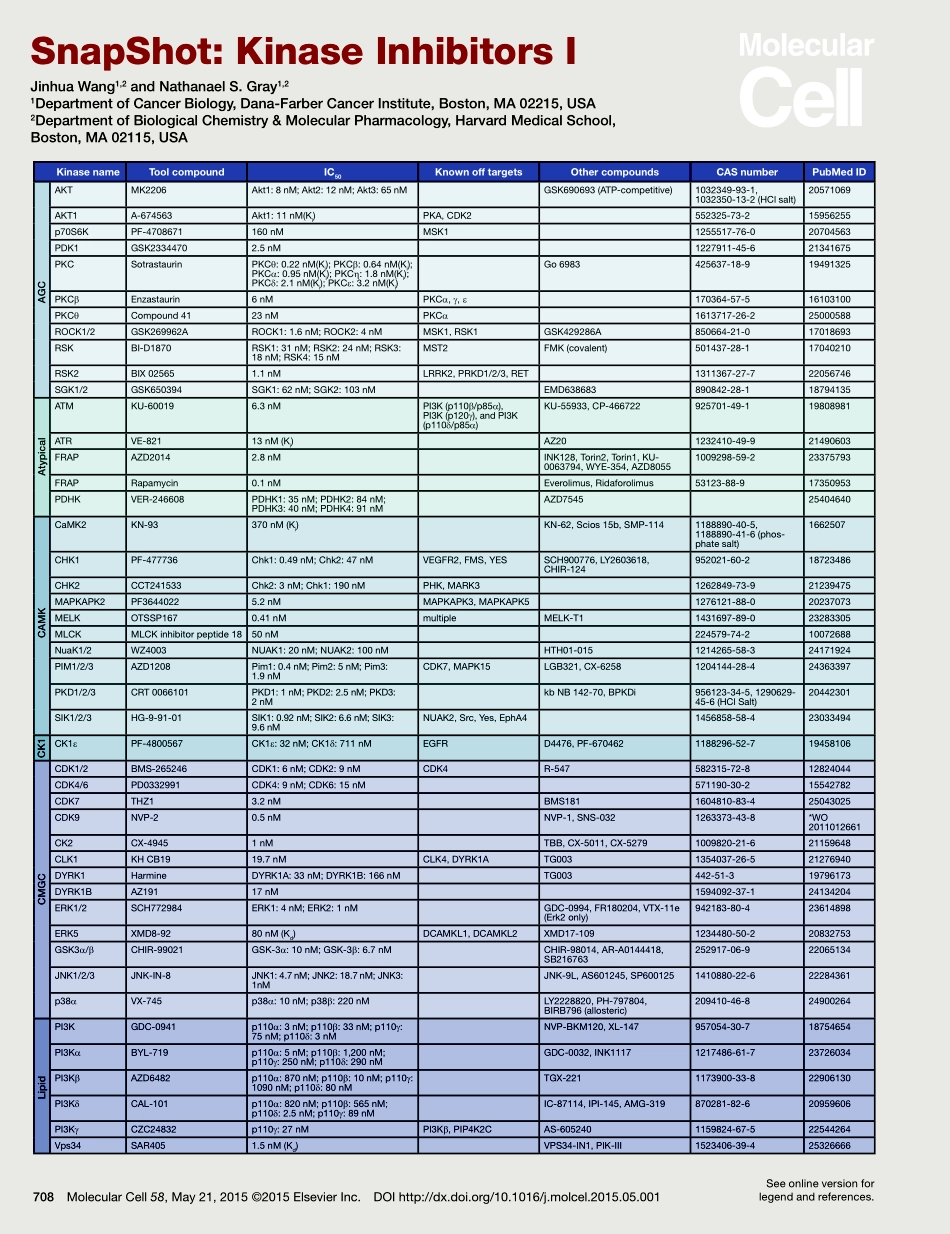
708MolecularCell58,May21,2015©2015ElsevierInc.DOIhttp://dx.doi.org/10.1016/j.molcel.2015.05.001SnapShot:KinaseInhibitorsIJinhuaWang1,2andNathanaelS.Gray1,21DepartmentofCancerBiology,Dana-FarberCancerInstitute,Boston,MA02215,USA2DepartmentofBiologicalChemistry&MolecularPharmacology,HarvardMedicalSchool,Boston,MA02115,USAKinasenameToolcompoundIC50KnownofftargetsOthercompoundsCASnumberPubMedIDAKTMK2206Akt1:8nM;Akt2:12nM;Akt3:65nMGSK690693(ATP-competitive)1032349-93-1,1032350-13-2(HClsalt)20571069AKT1A-674563Akt1:11nM(Ki)PKA,CDK2552325-73-215956255p70S6KPF-4708671160nMMSK11255517-76-020704563PDK1GSK23344702.5nM1227911-45-621341675PKCSotrastaurinPKCθ:0.22nM(Ki);PKCβ:0.64nM(Ki);PKCα:0.95nM(Ki);PKCη:1.8nM(Ki);PKCδ:2.1nM(Ki);PKCε:3.2nM(Ki)Go6983425637-18-919491325PKCβEnzastaurin6nMPKCα,g,ε170364-57-516103100PKCθCompound4123nMPKCα1613717-26-225000588ROCK1/2GSK269962AROCK1:1.6nM;ROCK2:4nMMSK1,RSK1GSK429286A850664-21-017018693RSKBI-D1870RSK1:31nM;RSK2:24nM;RSK3:18nM;RSK4:15nMMST2FMK(covalent)501437-28-117040210RSK2BIX025651.1nMLRRK2,PRKD1/2/3,RET1311367-27-722056746SGK1/2GSK650394SGK1:62nM;SGK2:103nMEMD638683890842-28-118794135ATMKU-600196.3nMPI3K(p110β/p85α),PI3K(p120g),andPI3K(p110δ/p85α)KU-55933,CP-466722925701-49-119808981ATRVE-82113nM(Ki)AZ201232410-49-921490603FRAPAZD20142.8nMINK128,Torin2,Torin1,KU-0063794,WYE-354,AZD80551009298-59-223375793FRAPRapamycin0.1nMEverolimus,Ridaforolimus53123-88-917350953PDHKVER-246608PDHK1:35nM;PDHK2:84nM;PDHK3:40nM;PDHK4:91nMAZD754525404640CaMK2KN-93370nM(Ki)KN-62,Scios15b,SMP-1141188890-40-5,1188890-41-6(phos-phatesalt)1662507CHK1PF-477736Chk1:0.49nM;Chk2:47nMVEGFR2,FMS,YESSCH900776,LY2603618,CHIR-124952021-60-218723486CHK2CCT241533Chk2:3nM;Chk1:190nMPHK,MARK31262849-73-921239475MAPKAPK2PF36440225.2nMMAPKAPK3,MAPKAPK51276121-88-020237073MELKOTSSP1670.41nMmultipleMELK-T11431697-89-023283305MLCKMLCKinhibitorpeptide1850nM224579-74-210072688NuaK1/2WZ4003NUAK1:20nM;NUAK2:100nMHTH01-0151214265-58-324171924PIM1/2/3AZD1208Pim1:...