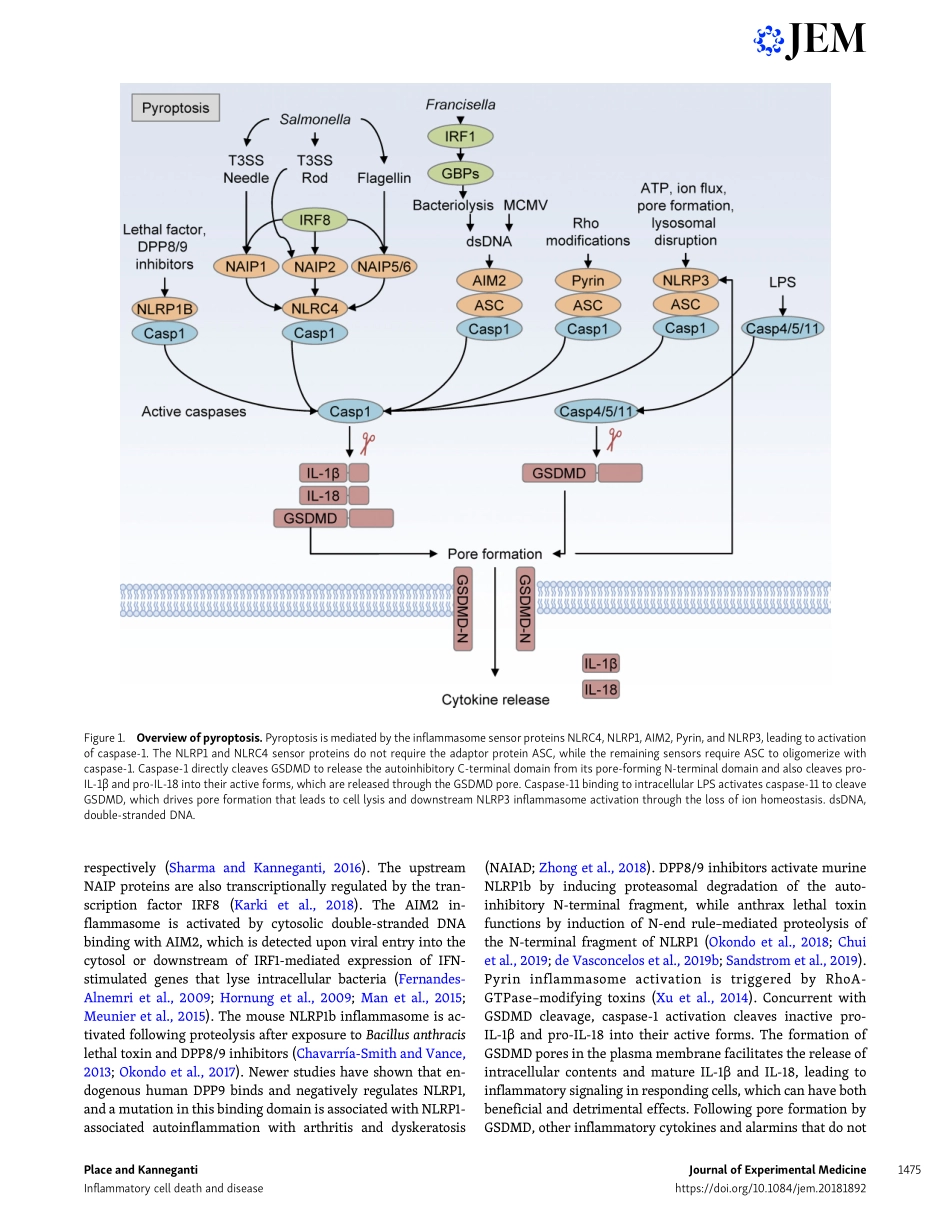
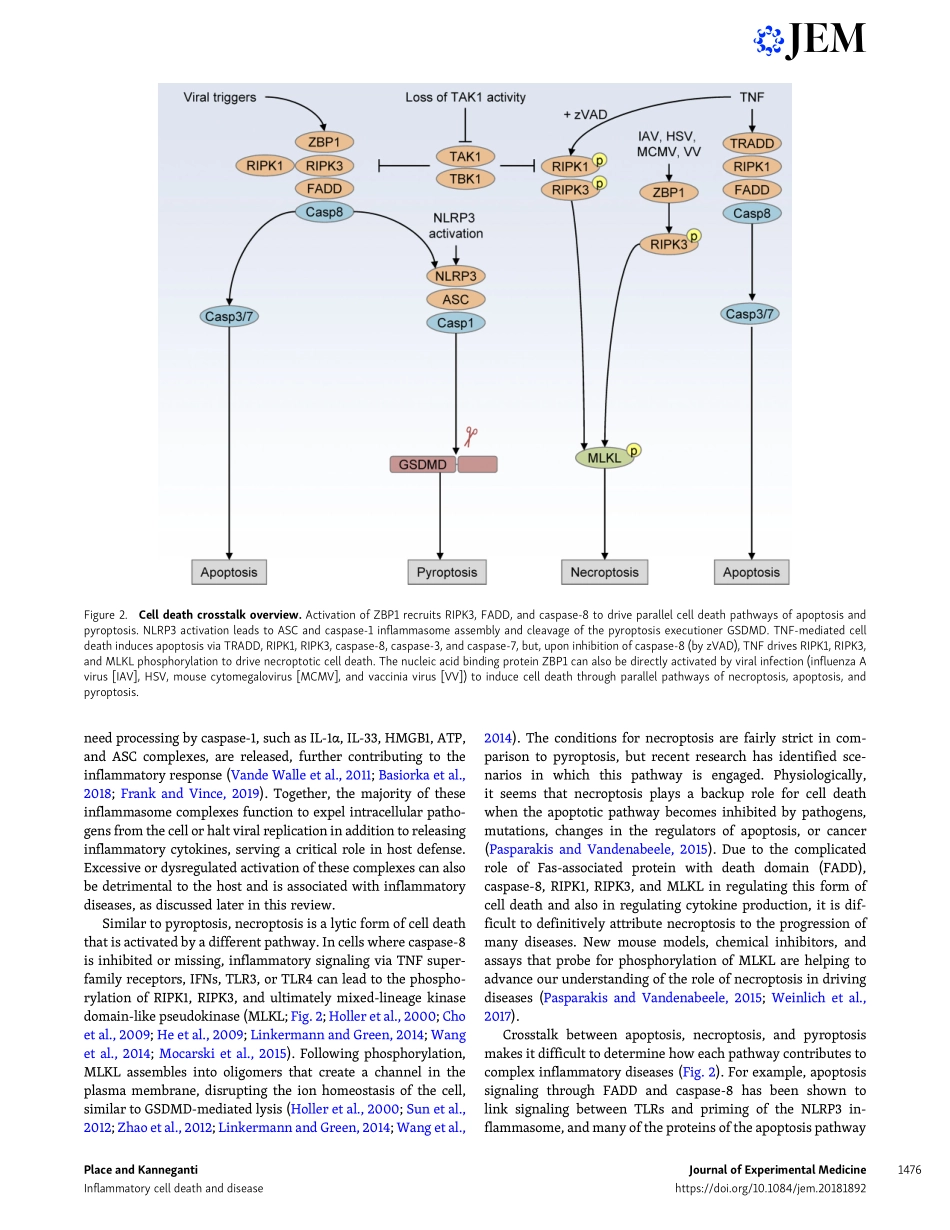
REVIEWCelldeath–mediatedcytokinereleaseanditstherapeuticimplicationsDavidE.PlaceandThirumala-DeviKannegantiTargetingapoptosistotreatdiseaseshasseentremendoussuccessoverthepastdecades.Morerecently,alternativeformsofregulatedcelldeath,includingpyroptosisandnecroptosis,havebeendescribed.Understandingthemolecularcascadesregulatingbothpyroptosisandnecroptosiswillyieldevenmoretargetstotreatdiseases.Theselyticformsofcelldeatharedistinctfromapoptosisduetotheircharacteristiclysisandreleaseofcellularcomponentsthatpromotediseaseordirectabeneficialimmuneresponse.Inthisreview,wefocusonhowpyroptosisandnecroptosis,whichreleasepotentimmunecytokinessuchasIL-1andIL-18,contributetovariousdiseases.Wealsoconsidertheimportantrolethattheexecutionersofthesecelldeathpathways,GSDMDandMLKL,playintheprogressionofinflammatorydiseases.Crosstalkbetweenthedifferentcelldeathpathwayslikelyplaysamajorrolephysiologically.Newtherapeuticstrategiestargetingthesespecificmoleculesholdenormouspotentialformanaginginflammatorydiseases.IntroductionRegulatedcelldeathhasbeenunderstoodasaconceptfordec-ades,withapoptosisbeingthefirstwell-definedprocessinwhichcellsdismantlethemselvesinaprocessthatisgenerallyimmunologicallyquiet(Kerretal.,1972;Elmore,2007).Apo-ptosisisinducedhomeostaticallyanduponexposuretoawidevarietyofinsults,leadingtotheactivationofinitiatorcaspases(caspase-8,-9,-10)andeffectorcaspases(caspase-3,-6,-7),re-sultinginanonlyticcelldeathcharacterizedbymembraneblebbing,cellshrinkage,andchromosomalcondensation(Elmore,2007).Whileapoptosisfacilitatesthecontrolleddeg-radationofintracellularproteinsandorganelles,pyroptosisandnecroptosisleadtocelllysisandthereleaseofawiderangeofintracellularcomponentsandinflammatorycytokines.Wefocusinthisreviewonthelyticformsofcelldeath(pyroptosisandnecroptosis)andtheconsequencesoftheircytokinerelease,withaneyetowardnewwaysoftreatinginflammatorydiseases.Unlikeapoptoticcelldeath,inwhichplasmamembranein-tegrityismaintainedandintracellularcomponentsareseques-tered,pyrop...