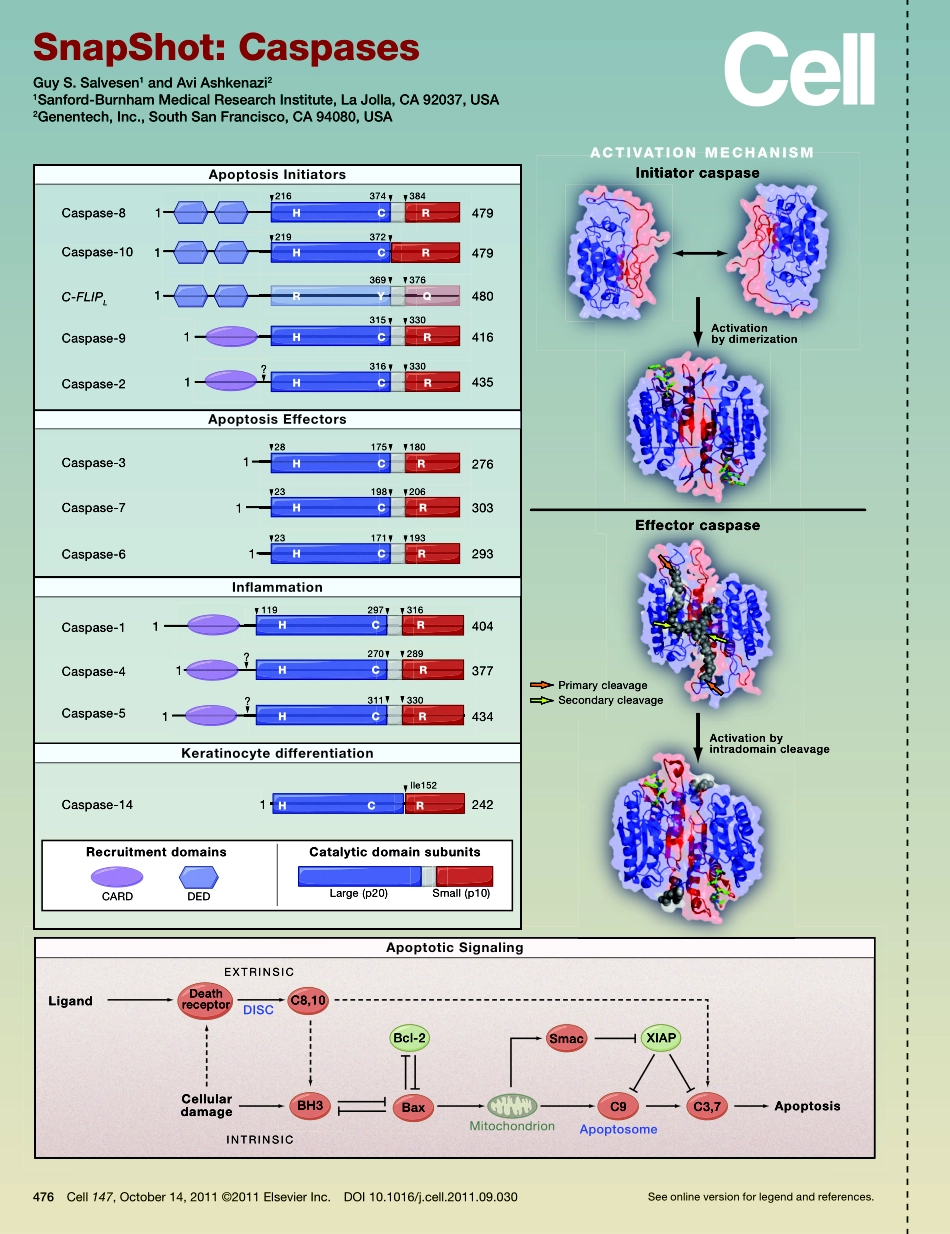
Caspase-8479479480416435276303293404377434242111111111111216219372369376315330316330175282323119?180198206171193297316270289311330Ile152374384LigandCellulardamageDISCEXTRINSICINTRINSICMitochondrionApoptosomeApoptosisInitiatorcaspaseEffectorcaspaseActivationbydimerizationActivationbyintradomaincleavagePrimarycleavageSecondarycleavageRecruitmentdomainsCARDDEDCatalyticdomainsubunitsLarge(p20)Small(p10)Caspase-9Caspase-2Caspase-3Caspase-1Caspase-14Caspase-4Caspase-5Caspase-7Caspase-6Caspase-10C-FLIPL384376384376216374374330330330330330315315315330330316316180180175281752062062319819819319323171171404316316119297297377289289270270434330242Ile152CatalyticdomainsubunitsSmall(p10)330Small(p10)CatalyticdomainsubunitsSmall(p10)3113111Ile152CatalyticdomainsubunitsLarge(p20)CatalyticdomainsubunitsCatalyticdomainsubunitsCatalyticdomainsubunits369369315315219372372219372372372RecruitmentdomainsDEDRecruitmentdomainsCARD1???HCHCRRRYQHCRHCRHCRHCRHCRHCRHCRHCRHCRC3,7ApoptosomeC9SmacXIAPBcl-2BaxBH3C8,10DISCEXTRINSICDeathreceptorApoptosisInitiatorsACTIVATIONMECHANISMApoptoticSignalingKeratinocytedifferentiationInflammationApoptosisEffectors476Cell147,October14,2011©2011ElsevierInc.DOI10.1016/j.cell.2011.09.030Seeonlineversionforlegendandreferences.SnapShot:CaspasesGuyS.Salvesen1andAviAshkenazi21Sanford-BurnhamMedicalResearchInstitute,LaJolla,CA92037,USA2Genentech,Inc.,SouthSanFrancisco,CA94080,USASnapShot:CaspasesGuyS.Salvesen1andAviAshkenazi21Sanford-BurnhamMedicalResearchInstitute,LaJolla,CA92037,USA2Genentech,Inc.,SouthSanFrancisco,CA94080,USA476.e1Cell147,October14,2011©2011ElsevierInc.DOI10.1016/j.cell.2011.09.030Caspases(cysteine-dependentaspartate-specificproteases)useaCyssidechaintocleaveAsp-containingpolypeptidesubstrates(Alnemrietal.,1996;Fuentes-PriorandSalvesen,2004).Theyperformselective,limitedcleavageofkeycellularsignalingcomponents.Caspasesplayimportantrolesinmetazoanembryogenesisandhomeostasis,regulatingdiversebiologicalprocessessuchasprogrammedcelldeath,inflammatio...