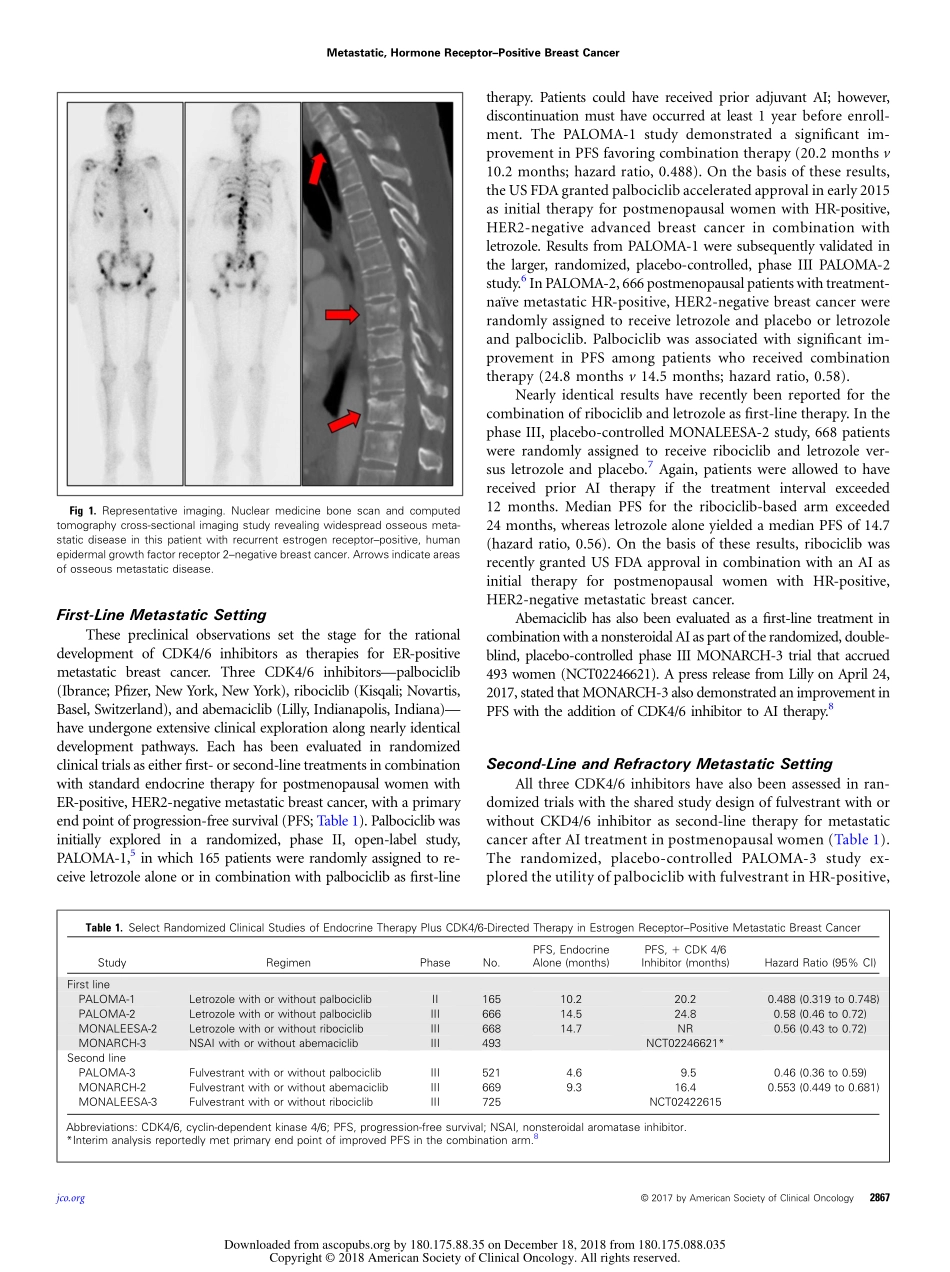
JOURNALOFCLINICALONCOLOGYONCOLOGYGRANDROUNDSBlockingtheCycle:Cyclin-DependentKinase4/6InhibitorsinMetastatic,HormoneReceptor–PositiveBreastCancerSethA.Wander,EricaL.Mayer,andHaroldJ.Burstein,Dana-FarberCancerInstitute;Brigham&Women’sHospital;HarvardMedicalSchool,Boston,MASeeaccompanyingarticleonpage2875TheOncologyGrandRoundsseriesisdesignedtoplaceoriginalreportspublishedintheJournalintoclinicalcontext.Acasepresentationisfollowedbyadescriptionofdiagnosticandmanagementchallenges,areviewoftherelevantliterature,andasummaryoftheauthors’suggestedmanagementapproaches.Thegoalofthisseriesistohelpreadersbetterunderstandhowtoapplytheresultsofkeystudies,includingthosepublishedinJournalofClinicalOncology,topatientsseenintheirownclinicalpractice.A68-year-oldpostmenopausalwomanwasdiagnosedwithbreastcancer6yearsagowhenshepresentedwithastageII(T2N1),right-sided,invasiveductalcarcinomaconsideredgrade2of3oncorebiopsy,withapositivefine-needleaspirationofapalpable,ipsilateralaxillarylymphnode.Immunohisto-chemicalanalysiswaspositiveforestrogenandprogesteronereceptorexpressionandnegativeforhumanepidermalgrowthfactorreceptor2(HER2)overexpression.Shereceivedneoadjuvantdose-densedoxorubicin,cyclophosphamide,andpaclitaxelchemotherapy,followedbybreast-conservingsurgeryandaxillarylymphnodedissection,whichrevealedresidualdiseaseinthreeof11nodes.Shereceivedadjuvantradiationtherapyandinitiatedletrozole,withexcellentcomplianceduringthein-terval6-yearperiod.Whilereceivingadjuvantletrozoletherapy,shereported3monthsofworseningbackpain.Skeletalscintigraphyandcross-sectionalimagingconfirmedwidespreadosseousmetastaticdiseaseandrightsupraclavicularlymphnodeenlargement(Fig1).Corebiopsyoftheinvolvedlymphnodeconfirmedestrogenreceptor(ER)–positive(90%),progesteronereceptor–negative,HER2-negativerecurrentmetastaticbreastcancer.Thepatientreportedmildpainthatwasadequatelycon-trolledwithover-the-counteranti-inflammatorymedications.Shehasremainedactivewithanexcellentperformancestatus.CHALLENGESINDIAGNOSISA...