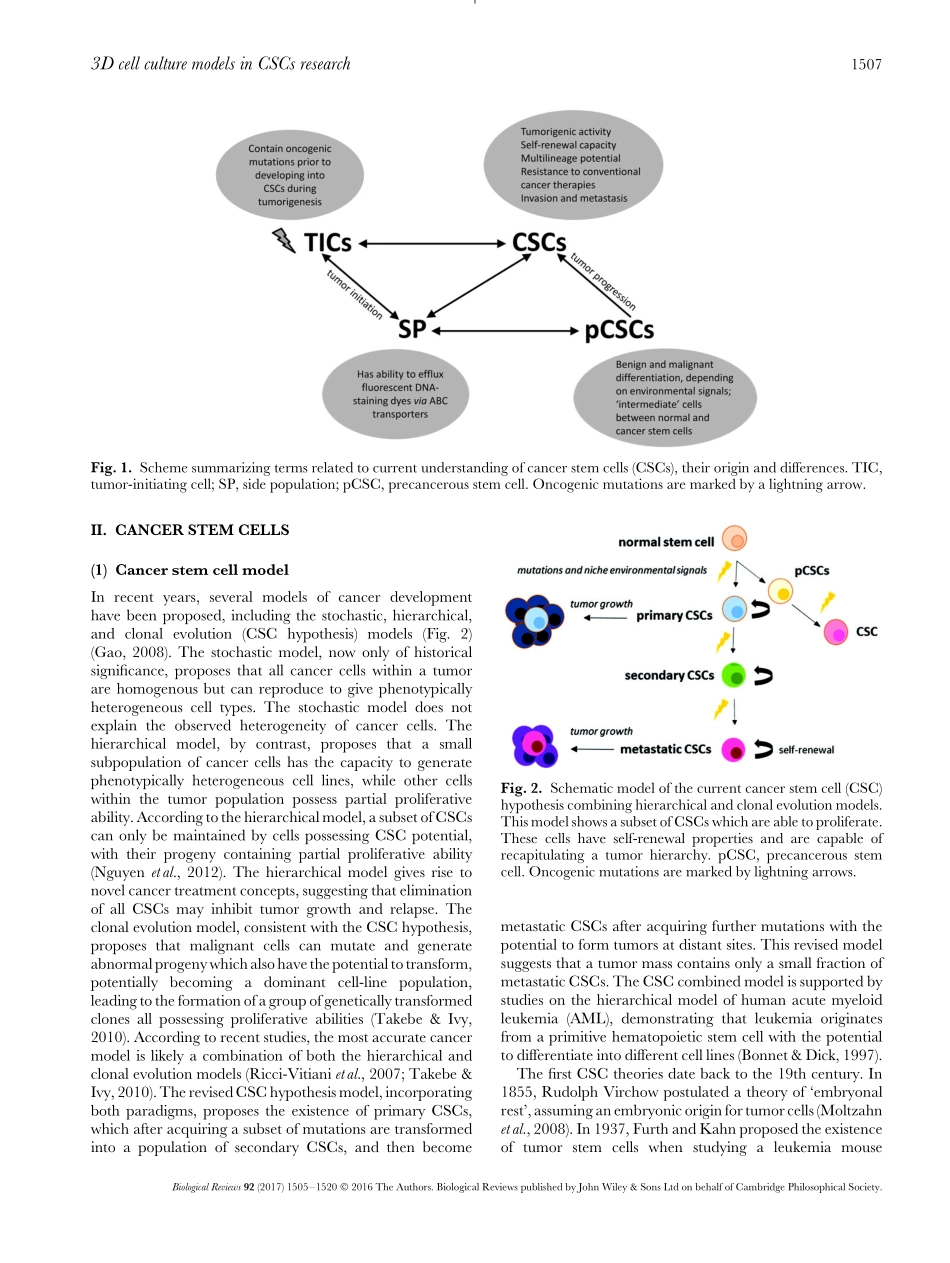
Biol.Rev.(2017),92,pp.1505–1520.1505doi:10.1111/brv.12293Three-dimensionalcellculturemodelutilizationincancerstemcellresearchZofiaF.Bielecka1,2,KamilaMaliszewska-Olejniczak1,3,IlanJ.Safir4,CezarySzczylik1andAnnaM.Czarnecka1,∗1DepartmentofOncologywithLaboratoryofMolecularOncology,MilitaryInstituteofMedicine,Szaser´ow128,04-141,Warsaw,Poland2PostgraduateSchoolofMolecularMedicine,MedicalUniversityofWarsaw,ZwirkiiWigury61,02-109,Warsaw,Poland3LaboratoryofDNASequencingandOligonucleotidesSynthesis,InstituteofBiochemistryandBiophysics,PolishAcademyofSciences,Pawinskiego5a,02-106Warsaw,Poland4DepartmentofUrology,EmoryUniversitySchoolofMedicine,Atlanta,GA30322,U.S.A.ABSTRACTThree-dimensional(3D)cellculturemodelsarebecomingincreasinglypopularincontemporarycancerresearchanddrugresistancestudies.Recently,scientistshavebegunincorporatingcancerstemcells(CSCs)into3Dmodelsandmodifyingculturecomponentsinordertomimicinvivoconditionsbetter.Currently,theglobalcellculturemarketisprimarilyfocusedoneither3Dcancercellculturesorstemcellcultures,withlessfocusonCSCs.Thisisevidentinthelowproductavailabilityofficiallyindicatedfor3DCSCmodelresearch.ThisreviewdiscussesthecurrentlyavailablecommercialproductsforCSC3Dculturemodelresearch.Additionally,wediscussdifferentculturemediaandcomponentsthatresultinhigherlevelsofstemcellsubpopulationswhilebetterrecreatingthetumormicroenvironment.Insummary,althoughprogresshasbeenmadeapplying3DtechnologytoCSCresearch,thistechnologycouldbefurtherutilizedandagreaternumberof3DkitsdedicatedspecificallytoCSCsshouldbeimplemented.Keywords:three-dimensionalsystems,cancerstemcells(CSCs),biomimiccultures,tumormicroenvironment,cancerniche.CONTENTSI.Introduction..............................................................................................1506II.Cancerstemcells.........................................................................................1507(1)Cancerstemcellmodel...............................................................................15...