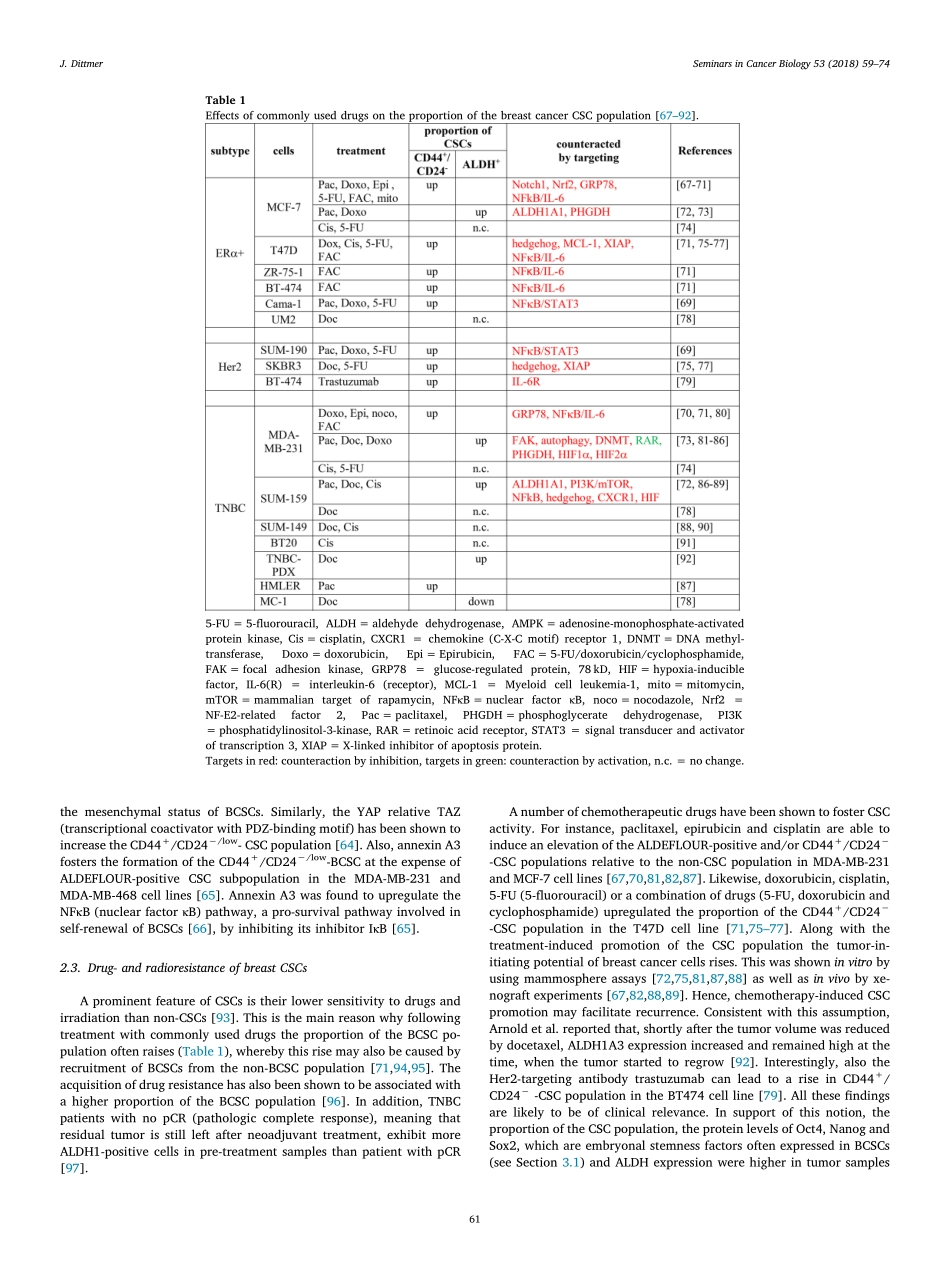
ContentslistsavailableatScienceDirectSeminarsinCancerBiologyjournalhomepage:www.elsevier.com/locate/semcancerBreastcancerstemcells:Features,keydriversandtreatmentoptionsJürgenDittmer⁎ClinicforGynecology,MartinLutherUniversityHalle-Wittenberg,GermanyARTICLEINFOKeywords:BreastcancerCancerstemcellsNotchWntHedgehogOSKMABSTRACTThecurrentviewisthatbreastcancerisastemcelldiseasecharacterizedbytheexistenceofcancercellswithstem-likefeaturesandtumor-initiatingpotential.Thesecellsaremaderesponsiblefortumordisseminationandmetastasis.Commontherapiesbychemotherapeuticdrugsfailtoeradicatethesecellsandratherincreasethepoolofcancerstemcellsintumors,aneffectthatmayincreasethelikelyhoodofrecurrence.Fifteenyearsafterthefirstevidenceforasmallstem-likesubpopulationplayingamajorroleinbreastcancerinitiationhasbeenpublishedalargebodyofknowledgehasbeenaccumulatedregardingthesignalingcascadesandproteinsin-volvedinmaintainingstemnessinbreastcancer.Differencesinthestemcellpoolsizeandinmechanismsregulatingstemnessinthedifferentbreastcancersubtypeshaveemerged.Overall,thisknowledgeoffersnewapproachestointervenewithbreastcancerstemcellactivity.Newoptionsareparticularlyneededforthetreatmentoftriple-negativebreastcancersubtype,whichisparticularlyrichincancerstemcellsandisalsothesubtypeforwhichspecifictherapiesarestillnotavailable.1.IntroductionBreastcanceristhemostfrequentcanceramongwomenworldwideandoveralltheleadingcauseofcancer-relateddeathinwomen[1].Breastcancerisnotahomogenousdisease.DifferentsubgroupscanbedistinguishedbyimmunohistochemistryandbymRNAanalysis.Im-munohistochemicalanalysisarecommonlyperformedtodeterminethestatusofERα(estrogenreceptorα),PR(progesteronereceptor),andHer2(humanepidermalreceptor2).Theyserveasbiomarkersfortreatment.ERα-positivetumorsareresponsivetoanti-estrogensandaromataseinhibitors,whichbothinterferewithERαfunction[2],Her2-positivetumorsreacttoHer2-directeddrugs,suchastheanti-Her2antibodytrastuzumab[3].Approximatelytwothirdofallbreastcan-cersexpressERαand∼15%...