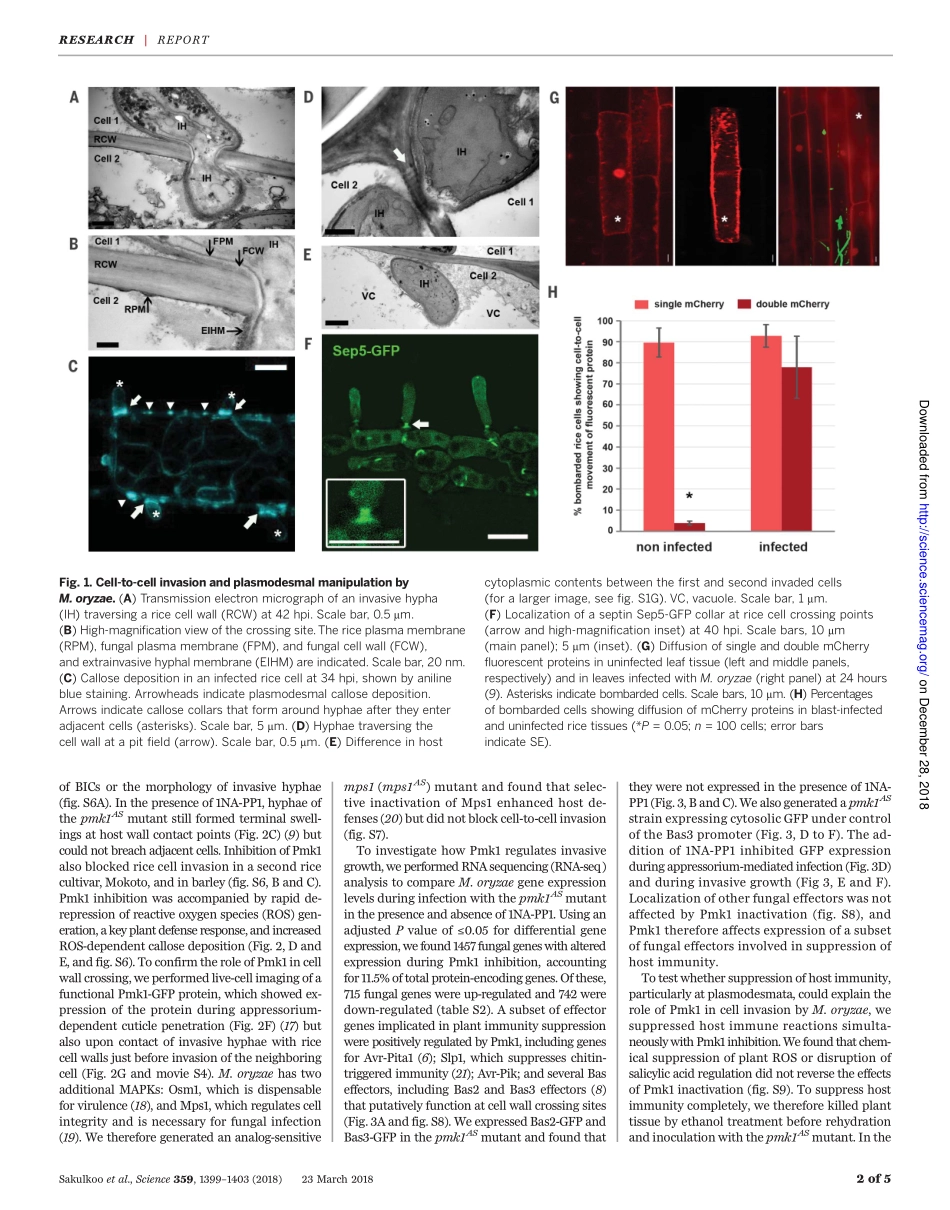
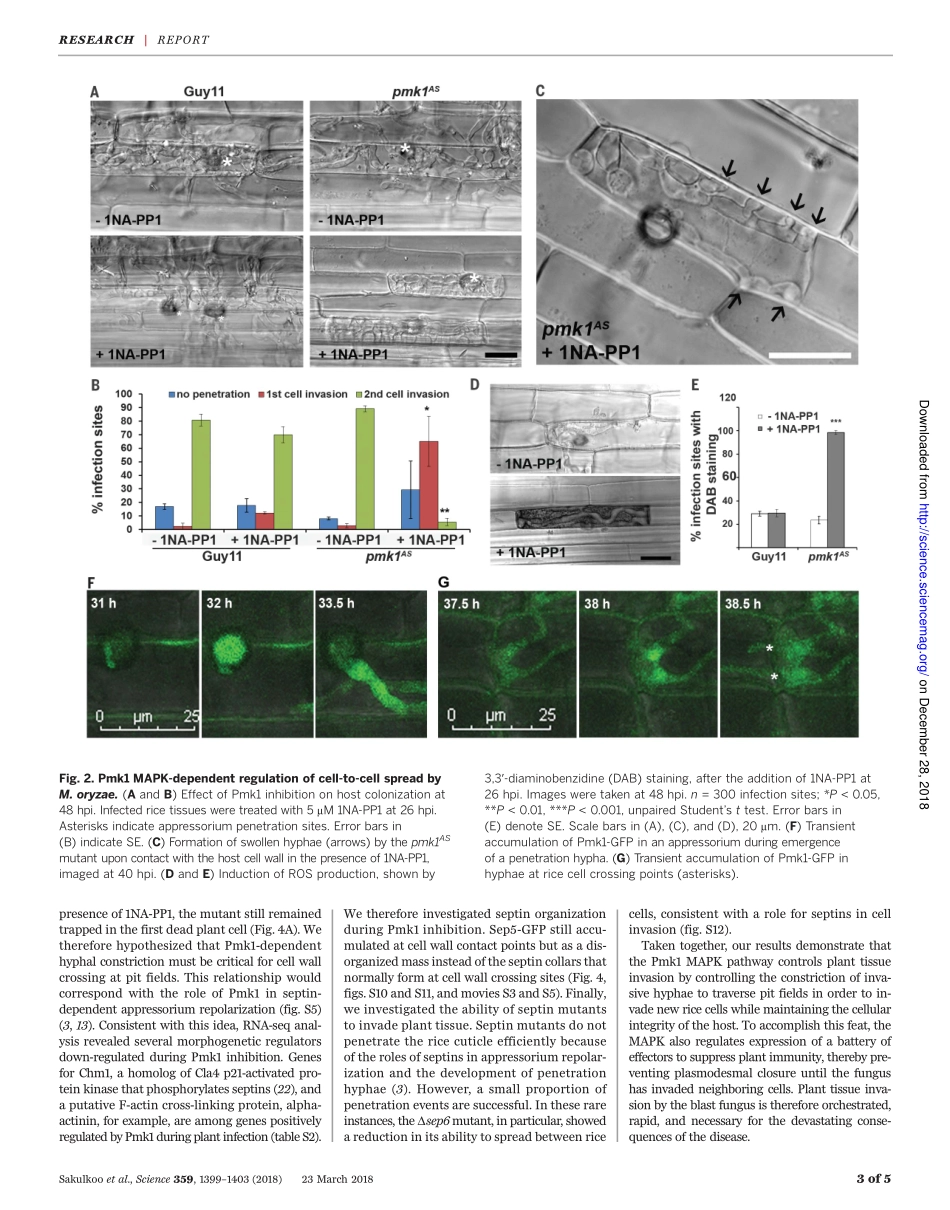
PLANTSCIENCEAsinglefungalMAPkinasecontrolsplantcell-to-cellinvasionbythericeblastfungusWasinSakulkoo,1MiriamOsés-Ruiz,1ElyOliveiraGarcia,2DarrenM.Soanes,1GeorgeR.Littlejohn,1*ChristianHacker,1AnaCorreia,1BarbaraValent,2NicholasJ.Talbot1†Blastdiseasedestroysupto30%ofthericecropannuallyandthreatensglobalfoodsecurity.TheblastfungusMagnaportheoryzaeinvadesplanttissuewithhyphaethatproliferateandgrowfromcelltocell,oftenthroughpitfields,whereplasmodesmatacluster.Weshowedthatchemicalgeneticinhibitionofasinglefungalmitogen-activatedprotein(MAP)kinase,Pmk1,preventsM.oryzaefrominfectingadjacentplantcells,leavingthefungustrappedwithinasingleplantcell.Pmk1regulatesexpressionofsecretedfungaleffectorproteinsimplicatedinsuppressionofhostimmunedefenses,preventingreactiveoxygenspeciesgenerationandexcessivecallosedepositionatplasmodesmata.Furthermore,Pmk1controlsthehyphalconstrictionrequiredforfungalgrowthfromonericecelltotheneighboringcell,enablinghosttissuecolonizationandblastdisease.BlastdiseasesofcerealsarecausedbythefilamentousfungusMagnaportheoryzae(synonymofPyriculariaoryzae),de-stroyingsufficientriceeachyeartofeed60millionpeople(1),andwheatblastdiseasenowthreatenswheatproductioninSouthAmericaand,mostrecently,Asia(2).Plantinfectionre-quiresaninfectioncell,calledanappressorium,whichusesapressure-drivenmechanismtobreachthetoughcuticleoftheleaf(3,4).Onceinsideplanttissue,thefunguselaboratespseudohypha-likeinvasivehyphaethatrapidlycolonizelivinghostcells,secretingeffectormoleculestosuppresshostimmunityandfacilitateinfection(5).M.oryzaeeffectorsaredeliveredintohostcytoplasmbymeansofabiotrophicinterfacialcomplex(BIC),aplant-derivedmembrane-richstructureinwhicheffectorsaccumulateduringtransittothehost(5–8).Hyphaethenappeartolocatepitfields,composedofplasmodesmata,whicharetraversedbyconstricted,narrowhyphae,enablingthespreadofthefungustoadjacenthostcells(9).Thefungusrapidlycolonizeshosttissue,anddiseaselesionsappearwithin4to5daysofinitialinfectionbyspores.Inthisstudy,weinvestigat...