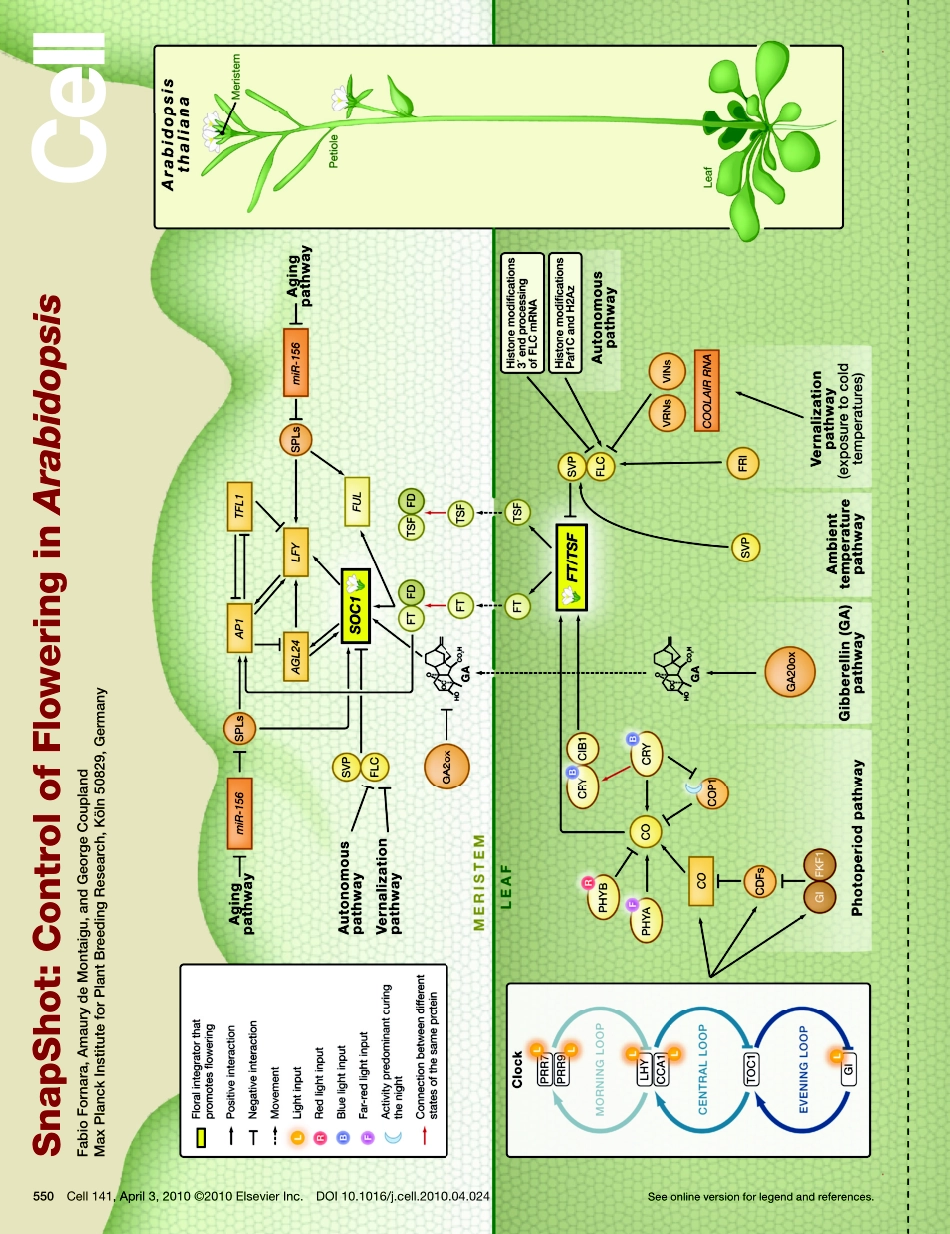
SnapShot:ControlofFloweringinArabidopsisFabioFornara,AmaurydeMontaigu,andGeorgeCouplandMaxPlanckInstituteforPlantBreedingResearch,Köln50829,GermanySeeonlineversionforlegendandreferences.550Cell141,April3,2010©2010ElsevierInc.DOI10.1016/j.cell.2010.04.024SnapShot:ControlofFloweringinArabidopsisFabioFornara,AmaurydeMontaigu,andGeorgeCouplandMaxPlanckInstituteforPlantBreedingResearch,Köln50829,Germany550.e1Cell141,April3,2010©2010ElsevierInc.DOI10.1016/j.cell.2010.04.024Plantsinitiatefloweringafteraperiodofvegetativedevelopment.Duringthisprocess,calledfloralinduction,theshootapicalmeristemstartstoproduceflowersinsteadofleaves.Thetimingoffloralinductioniscontrolledbysophisticatedregulatorynetworksthatmonitorchangesintheenvironment,ensuringthatfloweringoccursunderconditionsmostlikelytomaximizereproductivesuccessandseedproduction.InthemodelplantspeciesArabidopsisthaliana?180geneshavebeenimplicatedinflowering-timecontrolbasedonisolationofloss-of-functionmutationsoranalysisoftransgenicplants.ThisSnapShotpresentsasubsetofthesegenesandproteins,eachorganizedaccordingtoitsspatialactivityintheleavesortheshootapicalmeristemoftheplant.Strikingly,severalgenesactmorethanonceandinseveraltissuesduringfloralinduction.Manyofthesegenesoccurinanetworkofsixmajorpathways:thephotoperiodandvernalizationpathwayscontrolfloweringinresponsetoseasonalchangesindaylengthandtemperature;theambienttemperaturepathwayrespondstodailygrowthtemperatures;andtheage,autonomous,andgibberellinpathwaysactmoreindependentlyofenvironmentalstimuli.Thesixpathwaysconvergetoregulateasmallnumberof“floralintegratorgenes,”encodedbydifferentclassesofproteins,whichgovernfloweringtimebymergingsignalsfrommultiplepathways.TheseintegratorgenesincludeFLOWERINGLOCUST(FT)andSUPPRESSOROFOVEREXPRESSIONOFCONSTANS1(SOC1),whichbothrapidlypromotefloraldevelopment.Inaddition,responsestootherenvironmentalstimuli,suchasthebalanceofdifferentwavelengthsoflightornutrientavailability,alsoinfluencefloweringtime,buthowtheseprocessesinter...