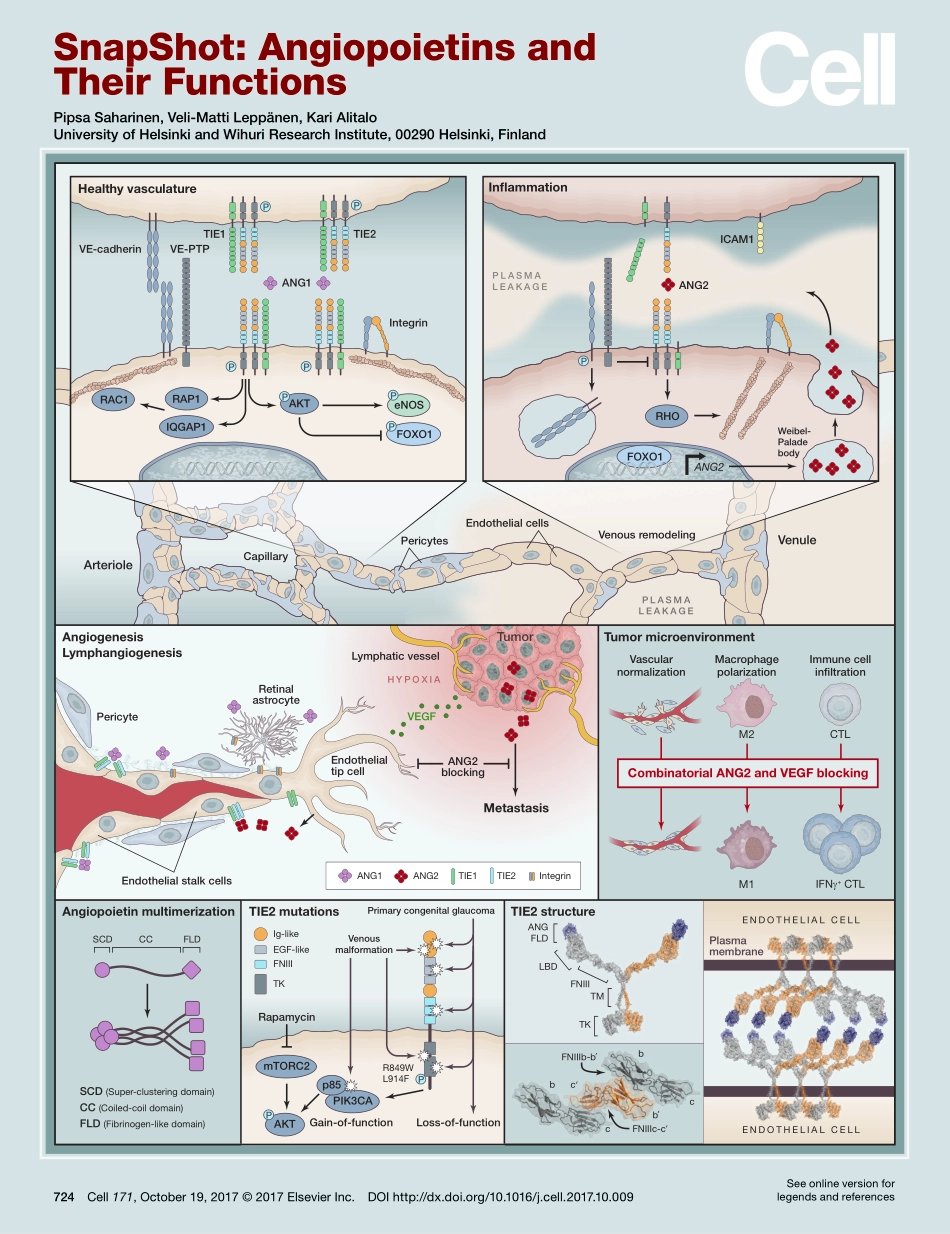
FOXO1HealthyvasculatureAngiogenesisLymphangiogenesisAngiopoietinmultimerizationSCD(Super-clusteringdomain)CC(Coiled-coildomain)FLD(Fibrinogen-likedomain)TIE2mutationsTIE2structureMetastasisRetinalastrocyteANG2blockingLymphaticvesselHYPOXIAVEGFEndothelialstalkcellsVE-cadherinVE-PTPTIE1PPPPPPPPPPANG1ANG1TIE1IntegrinTIE2ANG2Ig-likeCCFLDSCDEGF-likeANGFLDIFNγ+CTLENDOTHELIALCELLENDOTHELIALCELLEndothelialcellsEndothelialtipcellPlasmamembranePericytesPericyteCTLM2M1LBDFNIIIFNIIITMTKccbbPrimarycongenitalglaucomaVenousmalformationTKRapamycinR849WL914FmTORC2Loss-of-functionGain-of-functionp85PIK3CAAKTANG2ANG2ICAM1Weibel-PaladebodyRAC1ArterioleCapillaryRAP1AKTeNOSFOXO1PLASMALEAKAGEVenuleTumorTumormicroenvironmentVascularnormalizationImmunecellinfiltrationMacrophagepolarizationRHOIQGAP1IntegrinTIE2InflammationFNIIIc-cFNIIIb-bcbCombinatorialANG2andVEGFblockingVenousremodelingPLASMALEAKAGE724Cell171,October19,2017©2017ElsevierInc.DOIhttp://dx.doi.org/10.1016/j.cell.2017.10.009SeeonlineversionforlegendsandreferencesSnapShot:AngiopoietinsandTheirFunctionsPipsaSaharinen,Veli-MattiLeppänen,KariAlitaloUniversityofHelsinkiandWihuriResearchInstitute,00290Helsinki,Finland724.e1Cell171,October19,2017©2017ElsevierInc.DOIhttp://dx.doi.org/10.1016/j.cell.2017.10.009SnapShot:AngiopoietinsandTheirFunctionsPipsaSaharinen,Veli-MattiLeppänen,KariAlitaloUniversityofHelsinkiandWihuriResearchInstitute,00290Helsinki,FinlandAngiopoietins—MajorFunctionsTheangiopoietingrowthfactors(ANG1-4)bindtoendothelialTIE2receptortyrosinekinase,which,togetherwithligandlessTIE1receptor,formstheANG-TIEsignalingcomplexessentialforcardiovasculardevelopmentandforvenousandlymphaticvascularremodelinginanorganotypicmanner(AugustinandKoh,2017).ANG2expressionisincreasedinseveralhumandiseasesassociatedwithvasculardysfunction,suchassepsis,cancer,diabetes,majortraumas,malaria,andviralhemorrhagicfever(Saharinenetal.,2017).AngiopoietinSignalinginVesselStabilityandInflammationANG1stabilizesnewlyformedvessels,andANG1delivery...