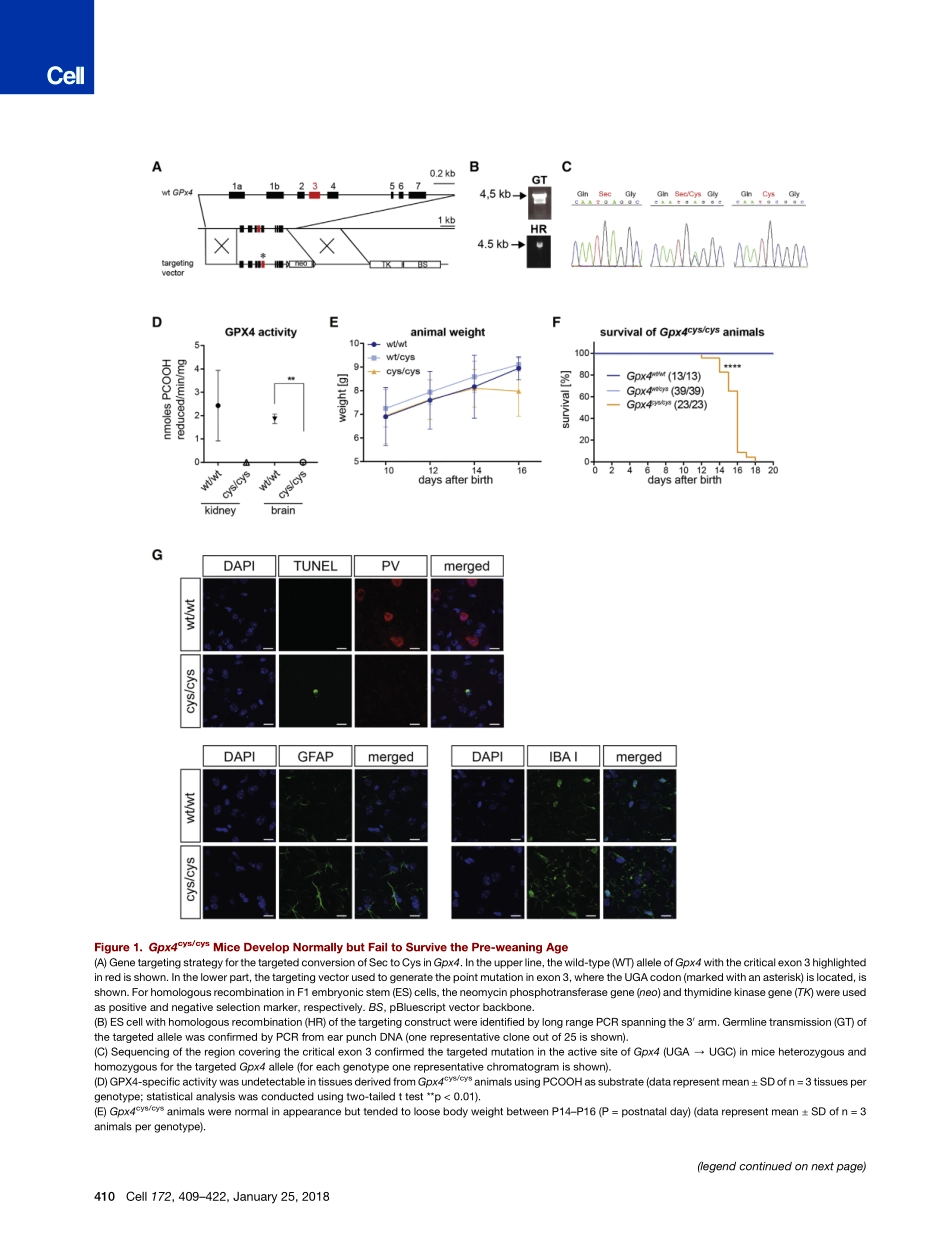
ArticleSeleniumUtilizationbyGPX4IsRequiredtoPreventHydroperoxide-InducedFerroptosisGraphicalAbstractHighlightsdSelenium-containingGPX4isnecessaryforfullviabilityofmicedTheGPX4-Cysvariantishighlysusceptibletohydroperoxide-inducedinactivationdHydroperoxideinducesferroptosisinGpx4cys/cyscellsdGPX4-CysbypassestherequirementofselenoproteinsforcellviabilityAuthorsIrinaIngold,CarstenBerndt,SabineSchmitt,...,HansZischka,Jose´PedroFriedmannAngeli,MarcusConradCorrespondencemarcus.conrad@helmholtz-muenchen.deInBriefThetraceelementseleniumprotectsacriticalpopulationofinterneuronsfromferroptoticcelldeath.Ingoldetal.,2018,Cell172,409–422January25,2018ª2017ElsevierInc.https://doi.org/10.1016/j.cell.2017.11.048ArticleSeleniumUtilizationbyGPX4IsRequiredtoPreventHydroperoxide-InducedFerroptosisIrinaIngold,1CarstenBerndt,2SabineSchmitt,3SebastianDoll,1GereonPoschmann,4KatalinBuday,1AntonellaRoveri,5XiaoxiaoPeng,6FlorencioPortoFreitas,1TobiasSeibt,7LisaMehr,1MichaelaAichler,8AxelWalch,8DanielLamp,9,10MartinJastroch,9,10SayuriMiyamoto,11WolfgangWurst,1,12,13FulvioUrsini,5EliasS.J.Arne´r,14NoeliaFradejas-Villar,15UlrichSchweizer,15HansZischka,3,16Jose´PedroFriedmannAngeli,1,17andMarcusConrad1,18,*1HelmholtzZentrumMu¨nchen,InstituteofDevelopmentalGenetics,85764Neuherberg,Germany2Heinrich-HeineUniversity,DepartmentofNeurology,MedicalFaculty,40255Du¨sseldorf,Germany3InstituteofToxicologyandEnvironmentalHygiene,TechnicalUniversityofMunich,80802Munich,Germany4Heinrich-HeineUniversity,MolecularProteomicsLaboratory,BiomedicalResearchCenter(BMFZ),40225Du¨sseldorf,Germany5DepartmentofMolecularMedicine,UniversityofPadova,Padova,Italy6CVMDTranslationalMedicineUnit,EarlyClinicalDevelopment,IMEDBiotechUnit,AstraZeneca,Gothenburg,Sweden7DepartmentofNephrology,MedizinischeKlinikundPoliklinikIV,KlinikumderUniversita¨tMu¨nchen,80336Mu¨nchen,Germany8HelmholtzZentrumMu¨nchen,ResearchUnitofAnalyticalPathology,85764Neuherberg,Germany9HelmholtzZentrumMu¨nchen,HelmholtzDiabetesCenterandGermanDi...