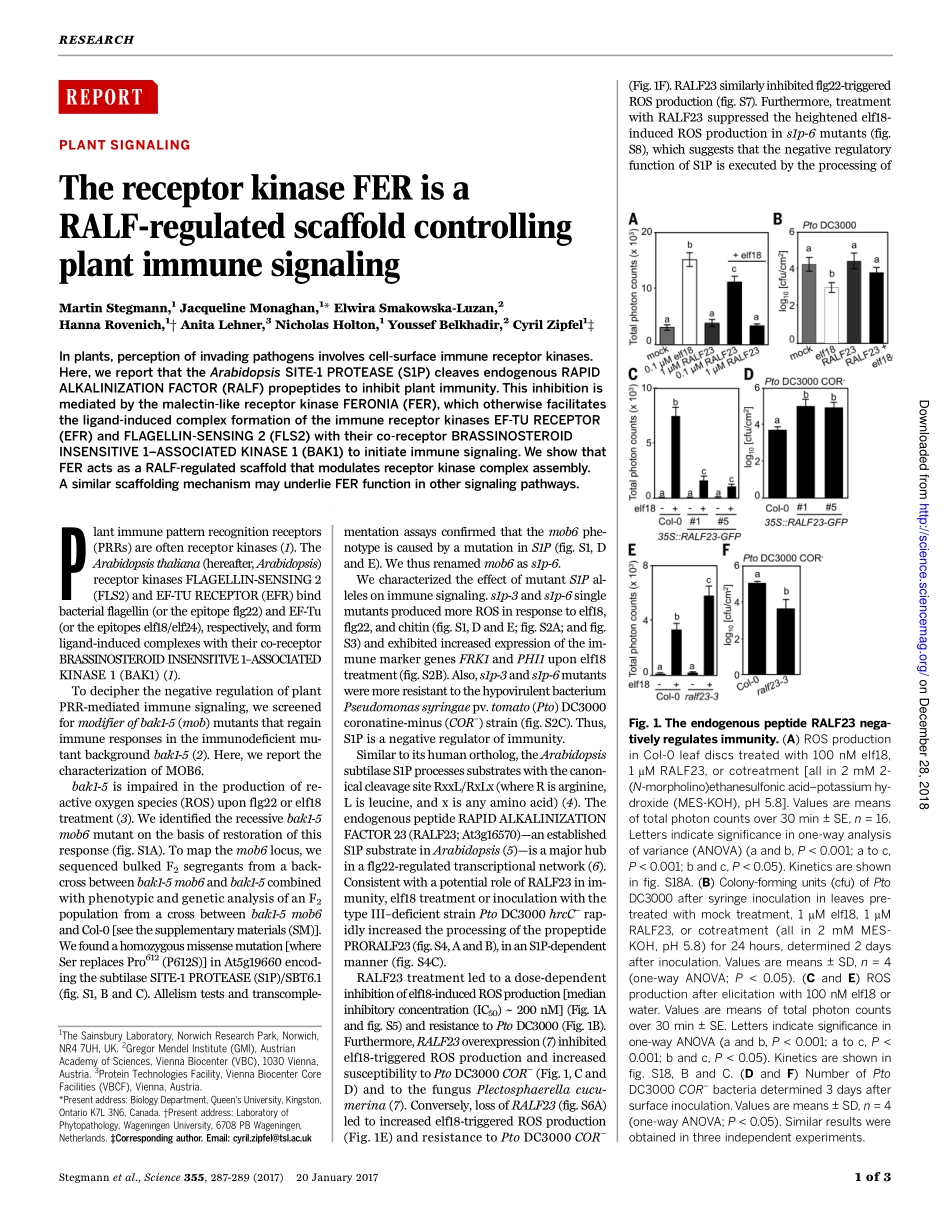
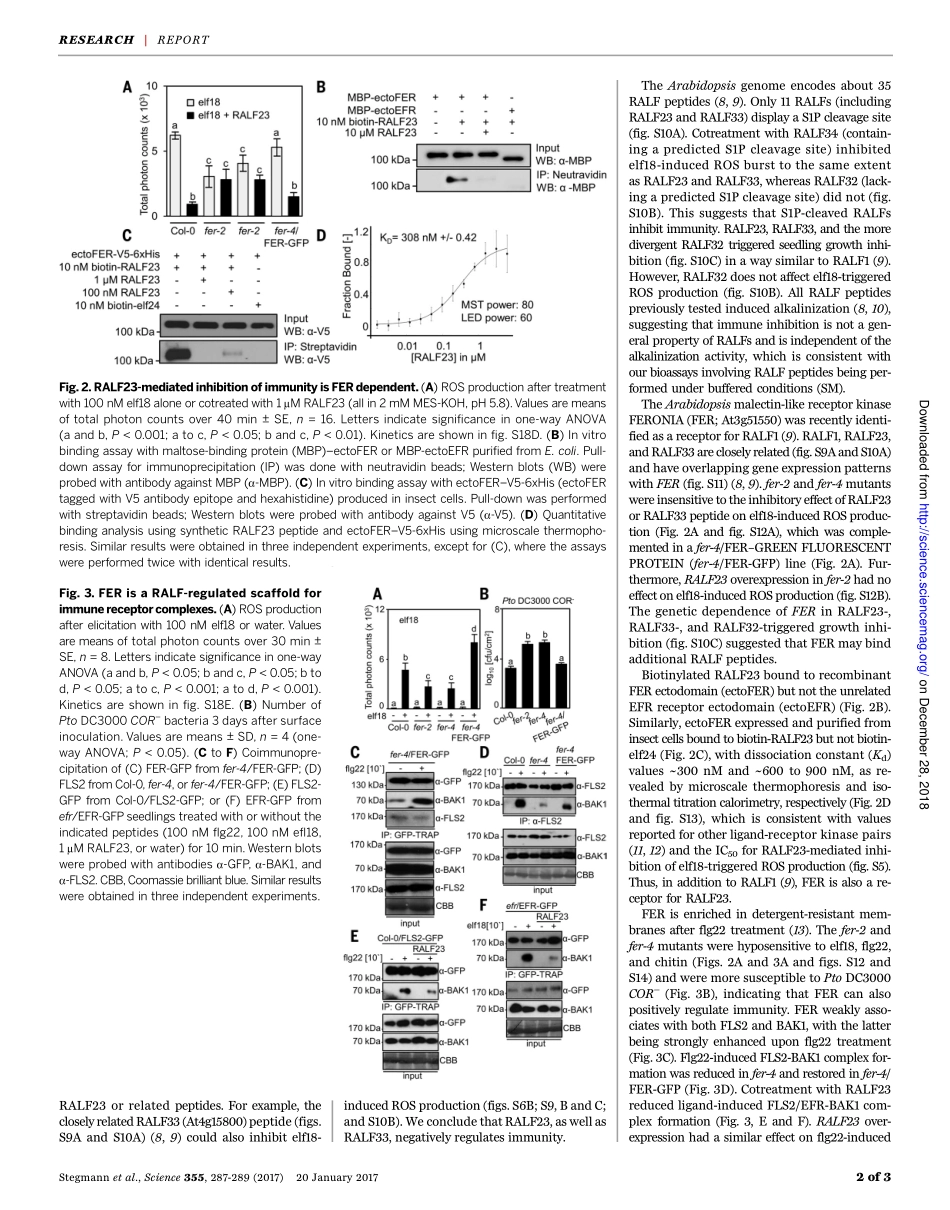
REPORT◥PLANTSIGNALINGThereceptorkinaseFERisaRALF-regulatedscaffoldcontrollingplantimmunesignalingMartinStegmann,1JacquelineMonaghan,1*ElwiraSmakowska-Luzan,2HannaRovenich,1†AnitaLehner,3NicholasHolton,1YoussefBelkhadir,2CyrilZipfel1‡Inplants,perceptionofinvadingpathogensinvolvescell-surfaceimmunereceptorkinases.Here,wereportthattheArabidopsisSITE-1PROTEASE(S1P)cleavesendogenousRAPIDALKALINIZATIONFACTOR(RALF)propeptidestoinhibitplantimmunity.Thisinhibitionismediatedbythemalectin-likereceptorkinaseFERONIA(FER),whichotherwisefacilitatestheligand-inducedcomplexformationoftheimmunereceptorkinasesEF-TURECEPTOR(EFR)andFLAGELLIN-SENSING2(FLS2)withtheirco-receptorBRASSINOSTEROIDINSENSITIVE1–ASSOCIATEDKINASE1(BAK1)toinitiateimmunesignaling.WeshowthatFERactsasaRALF-regulatedscaffoldthatmodulatesreceptorkinasecomplexassembly.AsimilarscaffoldingmechanismmayunderlieFERfunctioninothersignalingpathways.Plantimmunepatternrecognitionreceptors(PRRs)areoftenreceptorkinases(1).TheArabidopsisthaliana(hereafter,Arabidopsis)receptorkinasesFLAGELLIN-SENSING2(FLS2)andEF-TURECEPTOR(EFR)bindbacterialflagellin(ortheepitopeflg22)andEF-Tu(ortheepitopeself18/elf24),respectively,andformligand-inducedcomplexeswiththeirco-receptorBRASSINOSTEROIDINSENSITIVE1–ASSOCIATEDKINASE1(BAK1)(1).TodecipherthenegativeregulationofplantPRR-mediatedimmunesignaling,wescreenedformodifierofbak1-5(mob)mutantsthatregainimmuneresponsesintheimmunodeficientmu-tantbackgroundbak1-5(2).Here,wereportthecharacterizationofMOB6.bak1-5isimpairedintheproductionofre-activeoxygenspecies(ROS)uponflg22orelf18treatment(3).Weidentifiedtherecessivebak1-5mob6mutantonthebasisofrestorationofthisresponse(fig.S1A).Tomapthemob6locus,wesequencedbulkedF2segregantsfromaback-crossbetweenbak1-5mob6andbak1-5combinedwithphenotypicandgeneticanalysisofanF2populationfromacrossbetweenbak1-5mob6andCol-0[seethesupplementarymaterials(SM)].Wefoundahomozygousmissensemutation[whereSerreplacesPro612(P612S)]inAt5g19660encod-ingthesubtilaseSITE-1PROTEASE(S1...