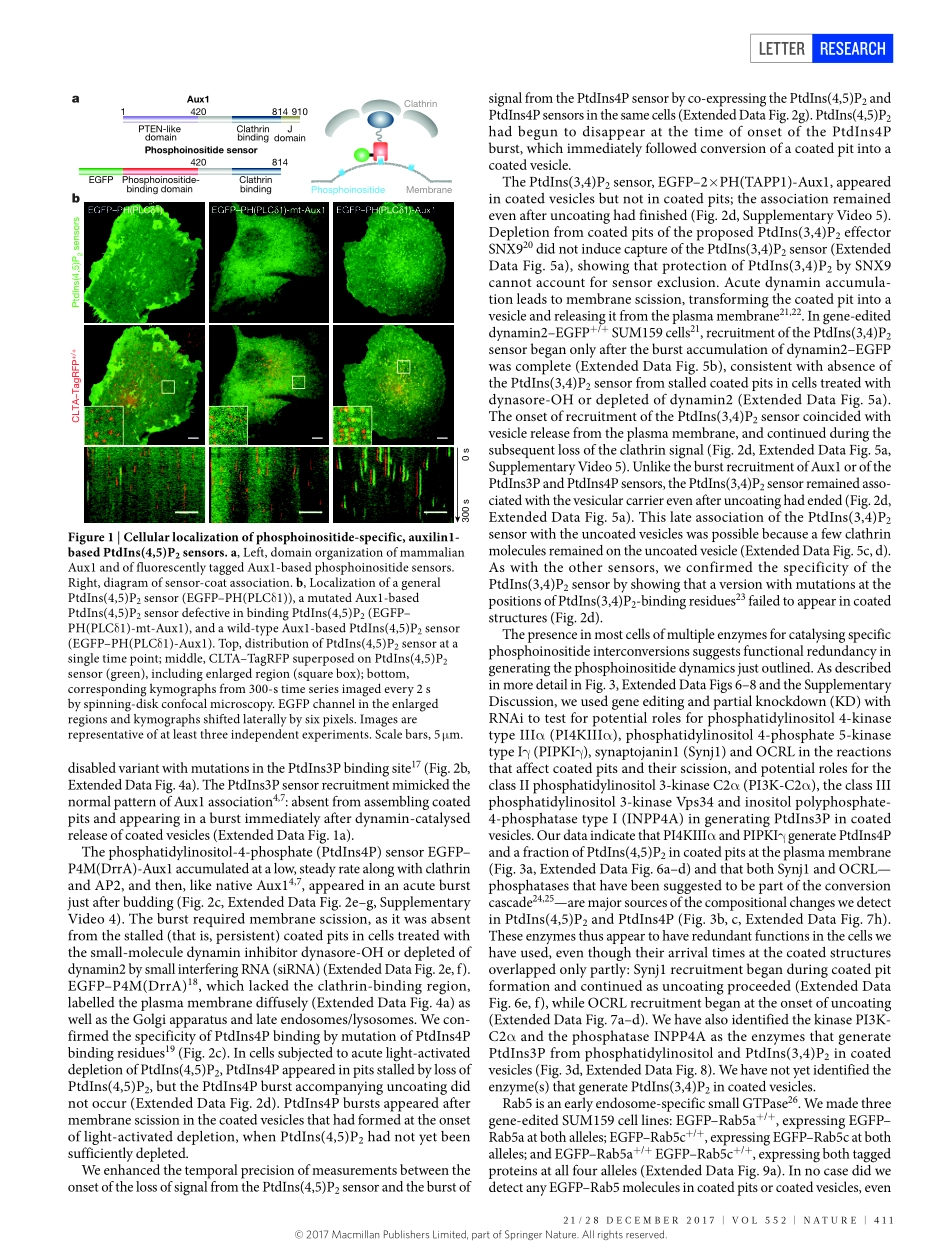
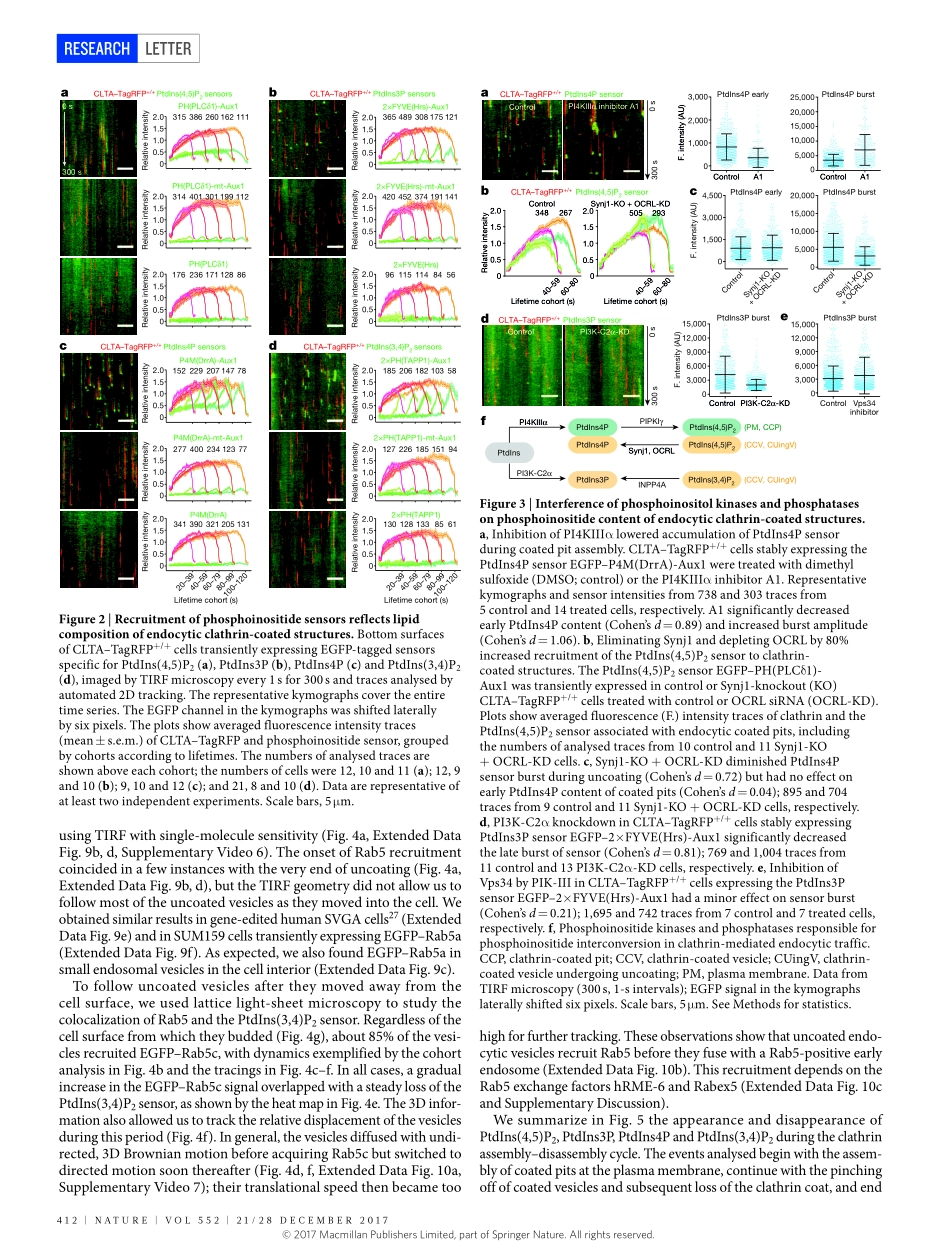
410|NATURE|VOL552|21/28DECEMBER2017LETTERdoi:10.1038/nature25146Dynamicsofphosphoinositideconversioninclathrin-mediatedendocytictrafficKangminHe1,2,3,RobertMarslandIII4†,SrigokulUpadhyayula1,2,3,EliSong2†,SongDang2,BenjaminR.Capraro1,2,WeimingWang1,2,WesleySkillern2,RaphaelGaudin1,2†,MingheMa2&TomKirchhausen1,2,3Vesicularcarrierstransportproteinsandlipidsfromoneorganelletoanother,recognizingspecificidentifiersforthedonorandacceptormembranes.TwoimportantidentifiersarephosphoinositidesandGTP-boundGTPases,whichprovidewell-definedbutmutablelabels.Phosphatidylinositolanditsphosphorylatedderivativesarepresentonthecytosolicfacesofmostcellularmembranes1,2.Reversiblephosphorylationofitsheadgroupproducessevendistinctphosphoinositides.Inendocytictraffic,phosphatidylinositol-4,5-biphosphatemarkstheplasmamembrane,andphosphatidylinositol-3-phosphateandphosphatidylinositol-4-phosphatemarkdistinctendosomalcompartments2,3.Itisunknownwhatsequenceofchangesinlipidcontentconfersonthevesiclestheirdistinctidentityateachintermediatestep.Herewedescribe‘coincidence-detecting’sensorsthatselectivelyreportthephosphoinositidecompositionofclathrin-associatedstructures,andtheuseofthesesensorstofollowthedynamicsofphosphoinositideconversionduringendocytosis.Themembraneofanassemblingcoatedpit,inequilibriumwiththesurroundingplasmamembrane,containsphosphatidylinositol-4,5-biphosphateandasmalleramountofphosphatidylinositol-4-phosphate.Closureofthevesicleinterruptsfreeexchangewiththeplasmamembrane.Asubstantialburstofphosphatidylinositol-4-phosphateimmediatelyafterbuddingcoincideswithaburstofphosphatidylinositol-3-phosphate,distinctfromanylaterencounterwiththephosphatidylinositol-3-phosphatepoolinearlyendosomes;phosphatidylinositol-3,4-biphosphateandtheGTPaseRab5thenappearandremainastheuncoatingvesiclesmatureintoRab5-positiveendocyticintermediates.Ourobservationsshowthatacascadeofmolecularconversions,madepossiblebytheseparationofavesiclefromitsparentmembrane,canlabelmembrane-trafficintermediatesan...