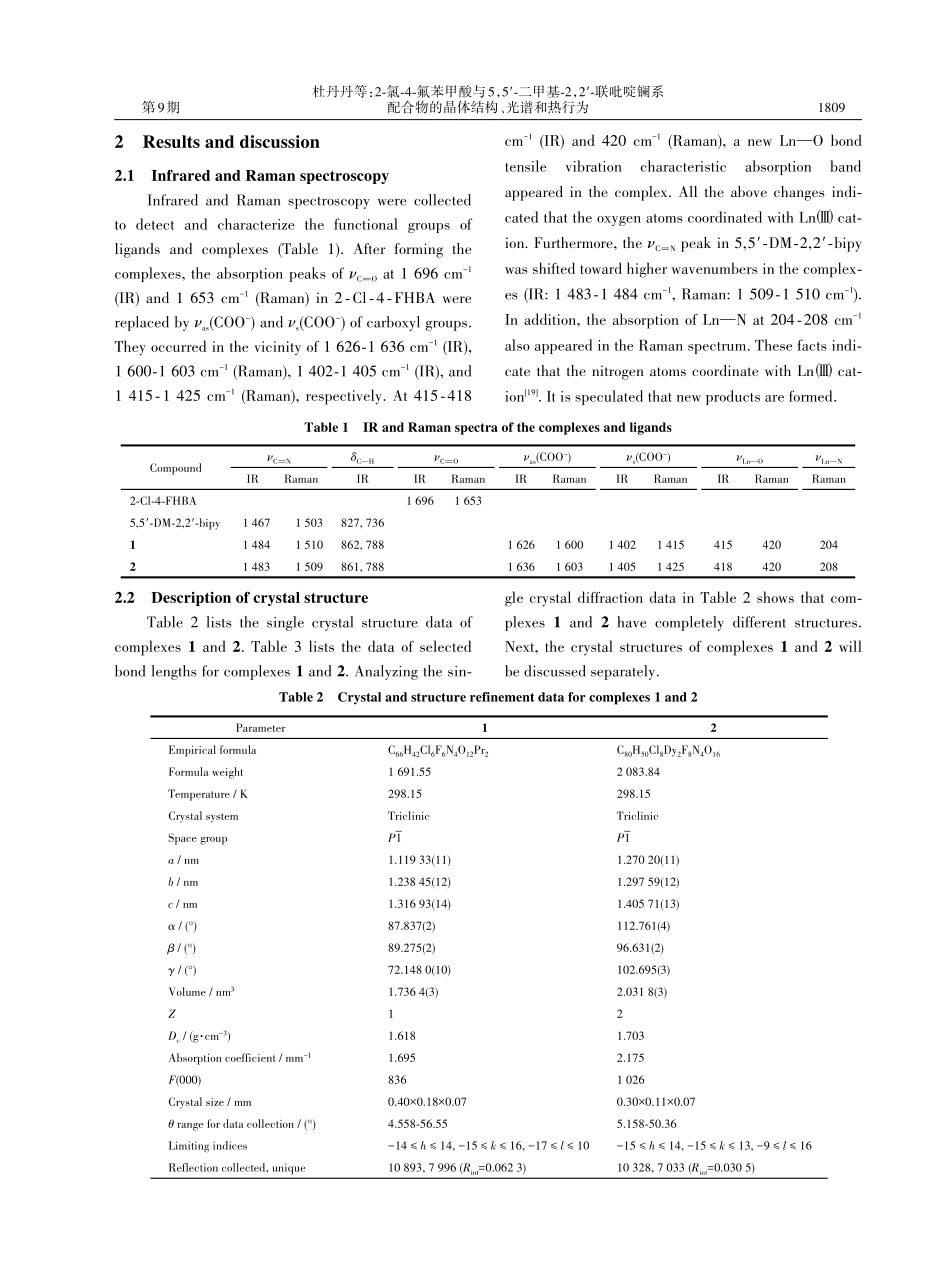
第39卷第9期2023年9月Vol.39No.91807⁃1816无机化学学报CHINESEJOURNALOFINORGANICCHEMISTRY收稿日期:2023⁃02⁃27。收修改稿日期:2023⁃06⁃24。国家自然科学基金(No.22273015)资助。*通信联系人。E⁃mail:jjzhang6@126.com,ningren9@163.com,zhaojinjin@hebtu.edu.cn2‑氯‑4‑氟苯甲酸与5,5′‑二甲基‑2,2′‑联吡啶镧系配合物的晶体结构、光谱和热行为杜丹丹1郝娅帆1王鑫鑫1赵金金*,1任宁*,2张建军*,1(1河北师范大学分析测试中心,化学与材料科学学院,石家庄050024)(2邯郸学院化学化工与材料学院,河北省杂环化合物重点实验室,邯郸056005)摘要:合成并表征了2个双核配合物[Pr(2⁃Cl⁃4⁃FBA)3(5,5'⁃DM⁃2,2'⁃bipy)]2(1)和[Dy(2⁃Cl⁃4⁃FBA)3(5,5'⁃DM⁃2,2'⁃bipy)]2·2(2⁃Cl⁃4⁃FHBA)(2),其中2⁃Cl⁃4⁃FHBA=2⁃氯⁃4⁃氟苯甲酸,5,5'⁃DM⁃2,2'⁃bipy=5,5'⁃二甲基⁃2,2'⁃联吡啶。配合物1以八配位的Pr3+为中心,其周围的配位环境为扭曲的三角十二面体。配合物2的结构是独特的,它包含2个自由的2⁃氯⁃4⁃氟苯甲酸分子,并以九配位的Dy3+为中心与周围的氮、氧原子形成扭曲的三棱镜几何构型。这2个配合物均结晶于三斜晶系P1空间群,并通过氢键相互作用和π⁃π堆积作用形成了一维和二维超分子结构。研究了配合物的热分解过程,结果表明配合物1和2分别分为4步和5步进行分解。同时对配合物的三维红外堆积图进行了研究,结果表明,整个热分解过程中释放出的主要气态产物是水、二氧化碳和有机小分子碎片。配合物2的荧光性质研究表明,它可以发射出Dy3+的特征跃迁对应的荧光。关键词:晶体结构;镧系配合物;热化学性质;光谱中图分类号:O614.33+4;O614.342文献标识码:A文章编号:1001⁃4861(2023)09⁃1807⁃10DOI:10.11862/CJIC.2023.138Crystalstructure,spectra,andthermalbehavioroflanthanidecomplexeswith2‑chloro‑4‑fluorobenzoicacidand5,5′‑dimethyl‑2,2′‑bipyridineDUDan⁃Dan1HAOYa⁃Fan1WANGXin⁃Xin1ZHAOJin⁃Jin*,1RENNing*,2ZHANGJian⁃Jun*,1(1TestingandAnalysisCenter,CollegeofChemistry&MaterialScience,HebeiNormalUniversity,Shijiazhuang050024,China)(2HebeiKeyLaboratoryofHeterocyclicCompounds,CollegeofChemicalEngineering&Material,HandanUniversity,Handan,Hebei056005,China)Abstract:Twobinuclearcomplexes[Pr(2⁃Cl⁃4⁃FBA)3(5,5'⁃DM⁃2,2'⁃bipy)]2(1)and[Dy(2⁃...