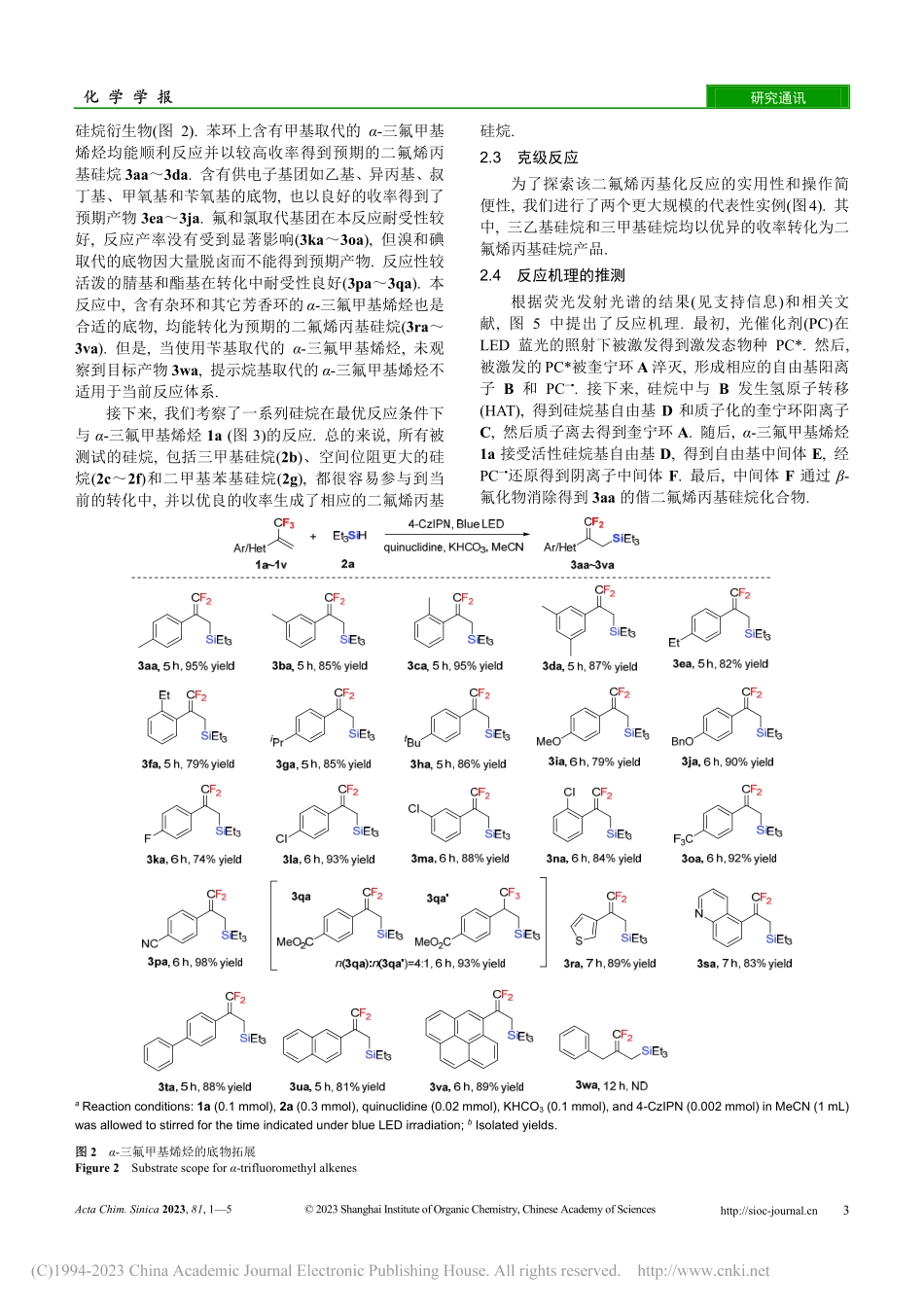
研究通讯Communication*E-mail:chenjingchao84@163.com;adams.bmf@hotmail.comReceivedNovember9,2022;publishedJanuary3,2023.SupportinginformationforthisarticleisavailablefreeofchargeviatheInternetathttp://sioc-journal.cn.ProjectsupportedbytheNationalNaturalScienceFoundationofChina(Nos.21961045,22061048)andYunnanProvincialKeyLaboratoryConstructionPlanFundingofUniversitiesandYunnanProvincialEngineeringResearchCenterConstructionPlanFundingofUniversities.项目受国家自然科学基金(Nos.21961045,22061048)及云南省高校重点实验室建设计划和云南省工程技术研究中心建设计划资助.ActaChim.Sinica2023,81,1—5©2023ShanghaiInstituteofOrganicChemistry,ChineseAcademyofScienceshttp://sioc-journal.cn1化学学报ACTACHIMICASINICA可见光催化的硅烷二氟烯丙基化反应杨春晖a陈景超*,a李新汉b孟丽b王凯民b孙蔚青b樊保敏*,a,b(a云南民族大学民族医药学院民族药资源化学国家民委-教育部重点实验室昆明650504)(b云南民族大学化学与环境学院云南民族大学高等合成化学重点实验室昆明650504)摘要研究了在蓝光照射下,以奎宁环作为氢原子转移试剂,实现了硅烷与α-三氟甲基烯烃的无金属二氟烯丙基化反应,并且通过使用多种芳香族和杂环α-三氟甲基烯烃,均能成功得到二氟烯丙基硅烷.本研究为制备二氟烯丙基硅烷提供了一种高效且经济的克级合成方法.关键词α-(三氟甲基)苯乙烯;硅烷;光催化;二氟烯丙基硅烷;奎宁环DifluoroallylationofSilanesunderPhotoirradiationYang,ChunhuiaChen,Jingchao*,aLi,XinhanbMeng,LibWang,KaiminbSun,WeiqingbFan,Baomin*,a,b(aSchoolofEthnicMedicine,KeyLaboratoryofChemistryinEthnicMedicinalResources,StateEthnicAffairsCommission&MinistryofEducation,YunnanMinzuUniversity,Kunming650504,China)(bSchoolofChemistryandEnvironment,KeyLaboratoryofAdvancedSyntheticChemistry,YunnanMinzuUniversity,Kunming650504,China)AbstractThesynthesisoforganosiliconcompoundshasattractedconsiderableinterest,especiallytheallylsilanes,whichareregardedasidealbuildingblocksinthesynthesisofsmallmoleculesandpolymers.Thetraditionalsyntheticmethodforallylsilanesreliesonthecross-couplingofGrignardreagentandchlorosilane(Silyl-Kumadareaction),transitionmetalcata-lyzedsilylationofallyl...