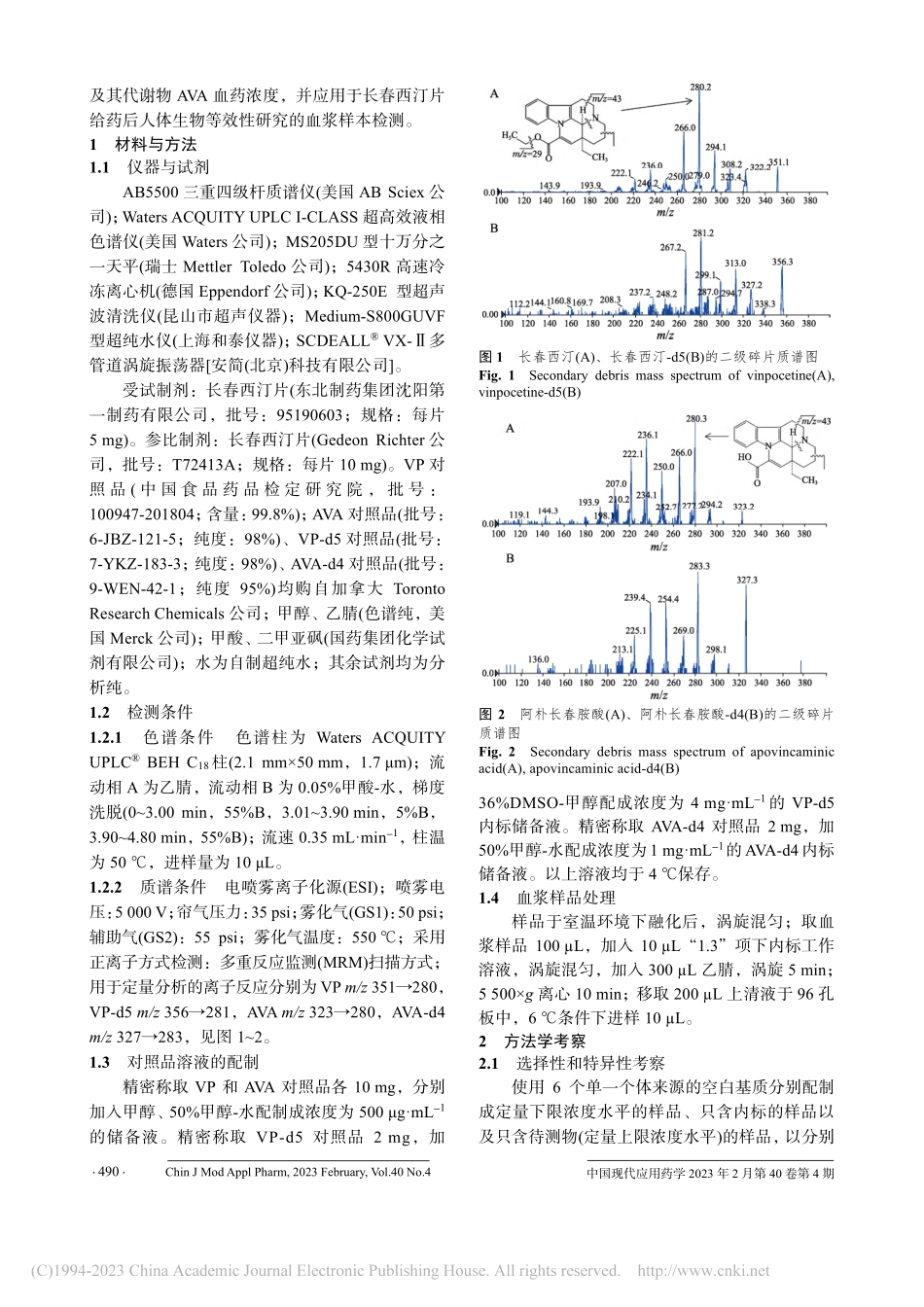
中国现代应用药学2023年2月第40卷第4期ChinJModApplPharm,2023February,Vol.40No.4·489·UPLC-MS/MS测定人血浆中长春西汀和阿朴长春胺酸的含量及其应用张林琪,苏文俏,郭建军*,游宇,胡林,周文(湖南恒兴医药科技有限公司,长沙410205)摘要:目的建立和验证UPLC-MS/MS测定人血浆中长春西汀和阿朴长春胺酸的方法,并用于临床样品检测。方法以长春西汀-d5、阿朴长春胺酸-d4为内标,人血浆样品经蛋白沉淀法沉淀处理,色谱柱为WatersACQUITYUPLC®BEHC18(2.1mm×50mm,1.7μm),流动相为乙腈-水(含0.05%甲酸),梯度洗脱,流速0.35mL·min−1;电喷雾离子化源正离子监测模式。结果长春西汀和阿朴长春胺酸线性范围分别为0.04~20.0ng·mL−1(r=0.9997)、0.50~250ng·mL−1(r=0.9996),检测限分别为0.01,0.10ng·mL−1,待测物与内标提取回收率均为94.81%~105.0%,基质效应为94.51%~105.0%,RSD均<5%;批内、批间精密度RSD均<10%。结论本法特异性强、快速、准确,重复性好,适用于长春西汀临床生物样品检测及生物等效性研究。关键词:长春西汀;阿朴长春胺酸;血药浓度;超高效液相色谱串联质谱法中图分类号:R917文献标志码:B文章编号:1007-7693(2023)04-0489-05DOI:10.13748/j.cnki.issn1007-7693.2023.04.009引用本文:张林琪,苏文俏,郭建军,等.UPLC-MS/MS测定人血浆中长春西汀和阿朴长春胺酸的含量及其应用[J].中国现代应用药学,2023,40(4):489-493.DeterminationofVinpocetineandApovincaminicAcidinHumanPlasmabyUPLC-MS/MSandItsApplicationZHANGLinqi,SUWenqiao,GUOJianjun*,YOUYu,HULin,ZHOUWen(EverProMedicalCo.,Ltd.,Changsha410205,China)ABSTRACT:OBJECTIVEToestablishandvalidateaUPLC-MS/MSforthedeterminationofvinpocetineandapovincaminicacidinhumanplasmaandapplytothedeterminationofclinicalsamples.METHODSVinpocetine-d5andapovincaminicacid-d4wereusedasinternalstandards.Humanplasmasampleswerepreparedwithacetonitriletoprecipitatetoprotein.ThechromatographiccolumnwasWatersACQUITYUPLC®BEHC18(2.1mm×50mm,1.7μm),themobilephasewasacetonitrile-water(containing0.05%formicacid).Gradientelutionwithaflowrateof0.35mL·min−1.Massspectrometryconditions:Electrosprayionizationsource(ESI),positiveiondetectionmode.RESULTSThelinearrangewere0.04−20.0ng·mL−1(r=0.9997)forvinpocetineand0.50−250...