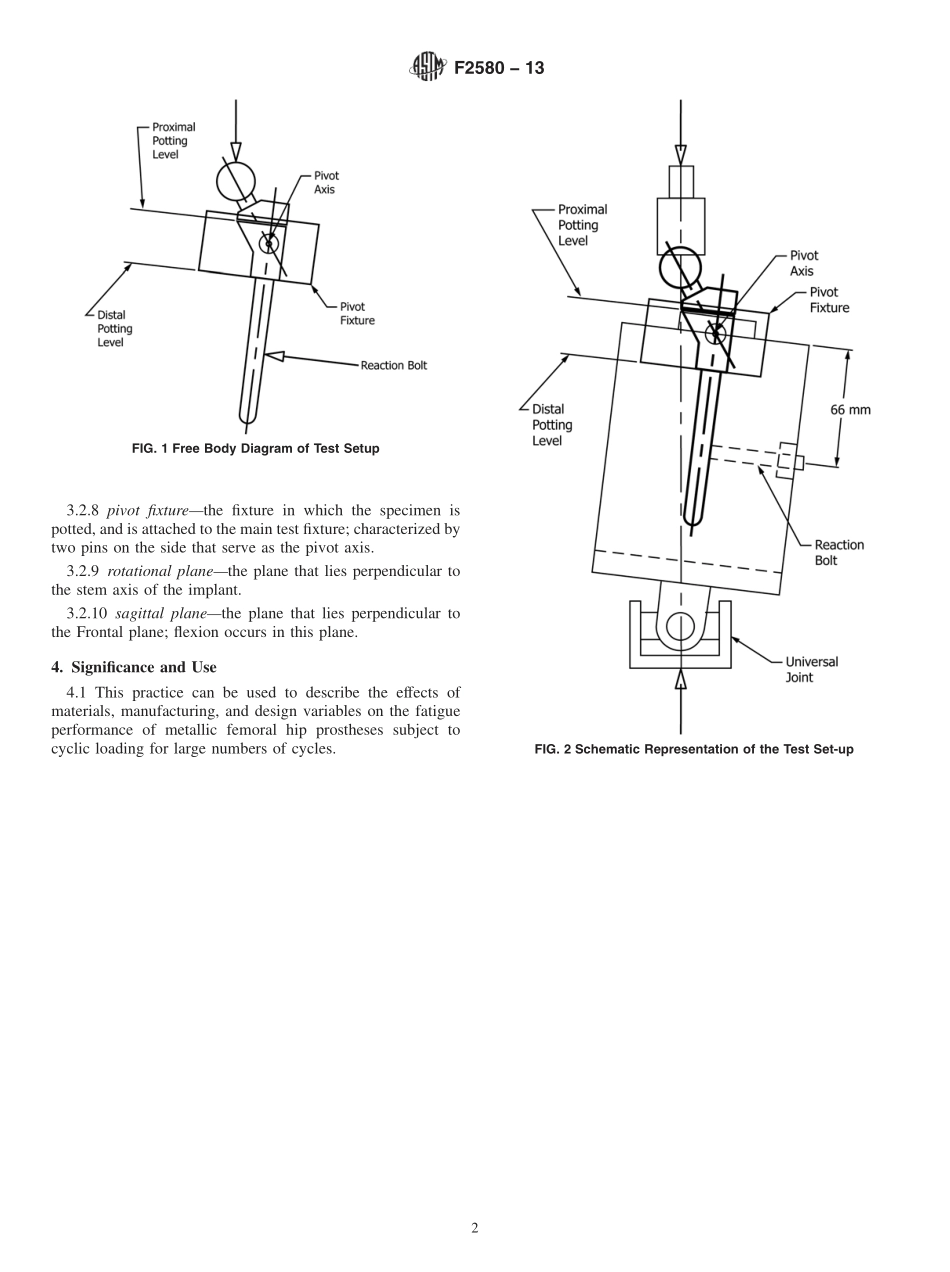
Designation:F2580−13StandardPracticeforEvaluationofModularConnectionofProximallyFixedFemoralHipProsthesis1ThisstandardisissuedunderthefixeddesignationF2580;thenumberimmediatelyfollowingthedesignationindicatestheyearoforiginaladoptionor,inthecaseofrevision,theyearoflastrevision.Anumberinparenthesesindicatestheyearoflastreapproval.Asuperscriptepsilon(´)indicatesaneditorialchangesincethelastrevisionorreapproval.1.Scope1.1Thispracticecoversaprocedureforthefatiguetestingofmetallicfemoralhipprosthesesusedinhipjointreplace-ments.Thispracticecoverstheproceduresfortheperformanceoffatiguetestsonmetallicfemoralhipstemsusingacyclic,constant-amplitudeforce.Itappliestohipprosthesesthatutilizeproximalmetaphysealfixationandareofamodularconstruct,anditisintendedtoevaluatethefatigueperformanceofthemodularconnectionsinthemetaphysealfilling(thatis,proximalbody)regionofthestem.1.2Thispracticeisintendedtoprovideuseful,consistent,andreproducibleinformationaboutthefatigueperformanceofmetallichipprostheseswhileheldinaproximallyfixatedmanner,withthedistalendnotheldbyapottingmedium.1.3ThevaluesstatedinSIunitsaretoberegardedasstandard.Nootherunitsofmeasurementareincludedinthisstandard.1.4Thisstandarddoesnotpurporttoaddressallofthesafetyconcerns,ifany,associatedwithitsuse.Itistheresponsibilityoftheuserofthisstandardtoestablishappro-priatesafetyandhealthpracticesanddeterminetheapplica-bilityofregulatorylimitationspriortouse.2.ReferencedDocuments2.1ASTMStandards:2E467PracticeforVerificationofConstantAmplitudeDy-namicForcesinanAxialFatigueTestingSystemE468PracticeforPresentationofConstantAmplitudeFa-tigueTestResultsforMetallicMaterialsE1150DefinitionsofTermsRelatingtoFatigue(Withdrawn1996)32.2ISOStandards:4ISO7206–4DeterminationofEndurancePropertiesofStemmedFemoralComponentswithApplicationofTor-sion3.Terminology3.1Definitions:3.1.1Rvalue,n—TheRvalueistheratiooftheminimumloadtothemaximumload.R5minimumloadmaximumload3.2DefinitionsofTermsSpecifictoThisStandard:3.2.1extraction—removalofthefemo...