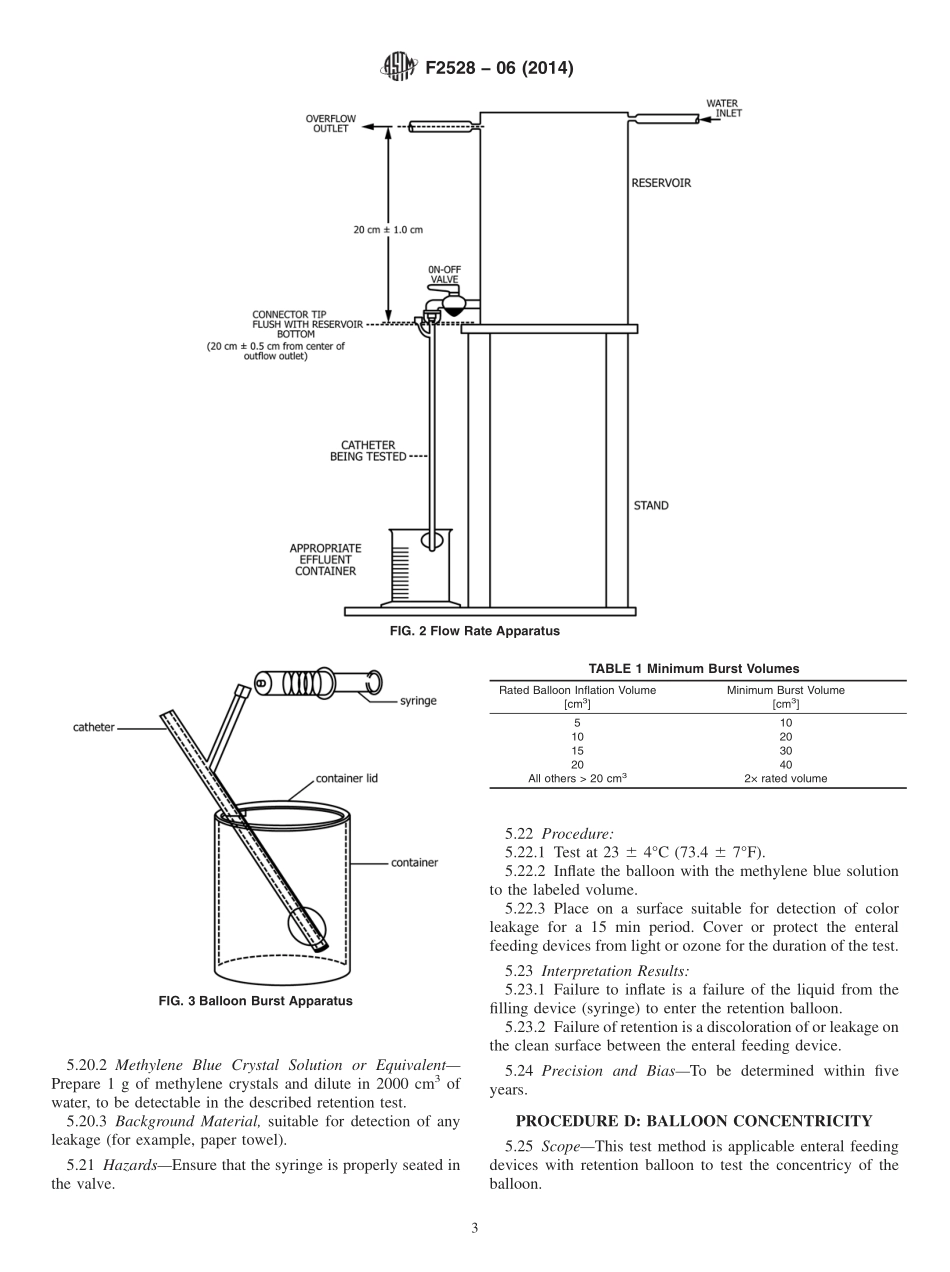
Designation:F2528−06(Reapproved2014)StandardTestMethodsforEnteralFeedingDeviceswithaRetentionBalloon1ThisstandardisissuedunderthefixeddesignationF2528;thenumberimmediatelyfollowingthedesignationindicatestheyearoforiginaladoptionor,inthecaseofrevision,theyearoflastrevision.Anumberinparenthesesindicatestheyearoflastreapproval.Asuperscriptepsilon(´)indicatesaneditorialchangesincethelastrevisionorreapproval.1.Scope1.1Thesetestmethodscovertheestablishmentofperfor-mancerequirementsfortheutilizationofasingle-use,enteralfeedingdevicewitharetentionballoon,usedbymedicalprofessionalsforprovidingameansofnutritionand/oradmin-istrationofmedicationtopatientsbymeansofnaturalorifice(nasal,oral,transluminal)andorasurgicallycreatedstoma.Theproductismanufacturedinvarioussizesandmaterialssuchassilicone,urethane,andvariouspolymers(aswellascombinationsofthese)andisprovidednonsterileforsteriliza-tionandsterileforsingleuseonly.RationaleforthesetestmethodscanbefoundinAppendixX1.1.2Thisstandarddoesnotpurporttoaddressallofthesafetyconcerns,ifany,associatedwithitsuse.Itistheresponsibilityoftheuserofthisstandardtoestablishappro-priatesafetyandhealthpracticesandtodeterminetheapplicabilityofregulatorylimitationspriortouse.2.ReferencedDocuments2.1ASTMStandards:2F623PerformanceSpecificationforFoleyCatheter2.2OtherStandard:SimulatedGastricFluid,USPOfficialCompendiaofStan-dards33.Terminology3.1Definitions:3.1.1balloonintegrity(resistancetorupture),n—volumeofliquidthatcorrespondswithballoonfailure,orbursting.3.1.2distal,n—referstotheballoonendoftheenteralfeedingdevice3.1.3enteralfeedingdevicewithretentionballoon,n—atwo-waymedicaldeviceintendedtoprovideameansofnutritionoradministrationofmedication,orboth,topatientsbymeansofnaturalorifice(nasal,oral,transluminal)orasurgicallycreatedstoma,orboth,consistingofadrainagelumenandinflationlumen(seeFig.1).Commonballooninflationsizesare5cm3,15cm3,and20cm3.3.1.4Frenchsize(Fr),n—ascaleusedfordenotingthesizeofcathetersandothertubularinstruments.TheFrenchsiz...