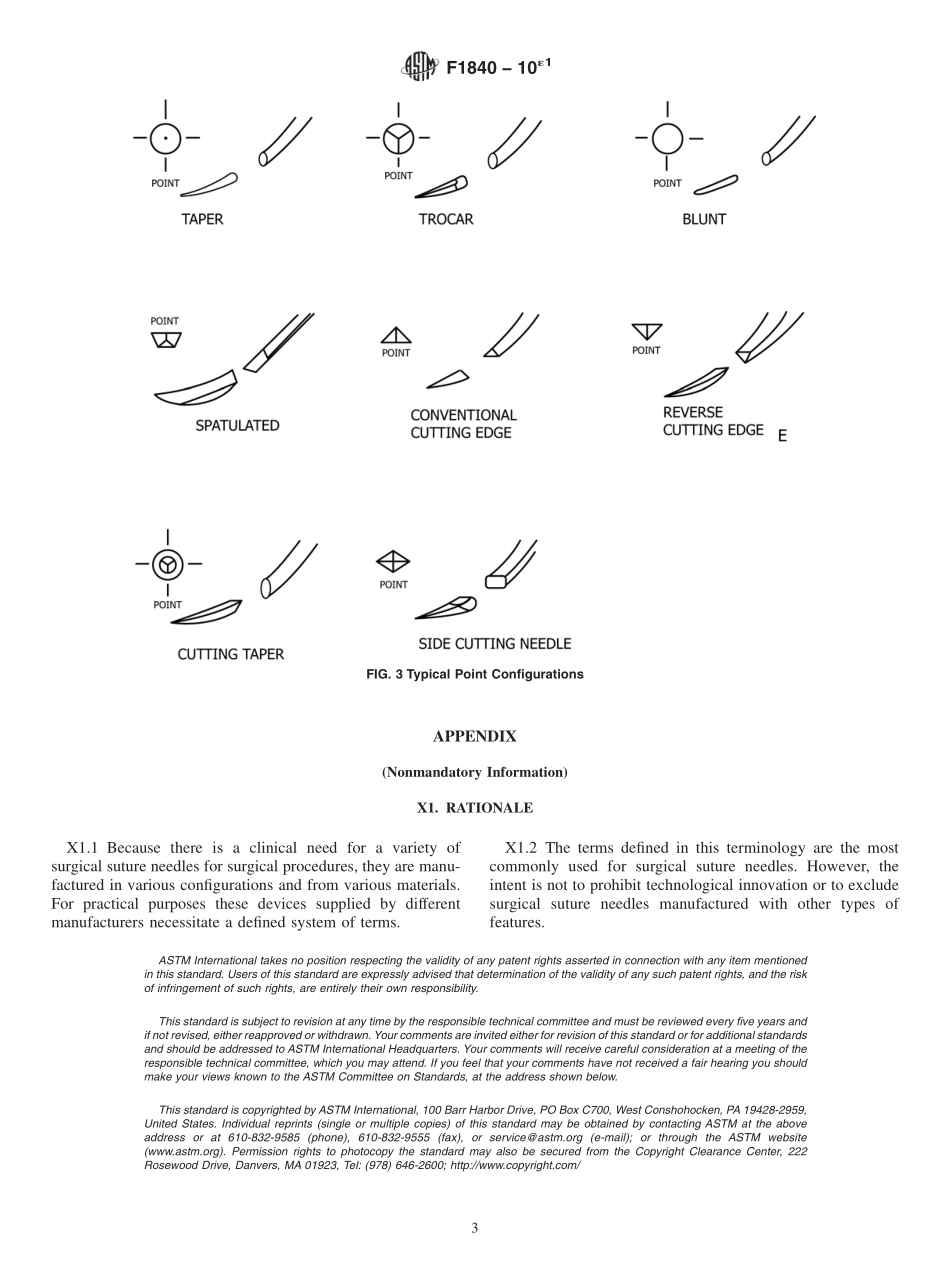
Designation:F1840−10´1StandardTerminologyforSurgicalSutureNeedles1ThisstandardisissuedunderthefixeddesignationF1840;thenumberimmediatelyfollowingthedesignationindicatestheyearoforiginaladoptionor,inthecaseofrevision,theyearoflastrevision.Anumberinparenthesesindicatestheyearoflastreapproval.Asuperscriptepsilon(´)indicatesaneditorialchangesincethelastrevisionorreapproval.ε1NOTE—“TypicalCurvatures”figurewaseditoriallyincludedinMay2011.1.Scope1.1Thisterminologycoversgeneraldefinitionsforsurgicalneedles.2.Terminology2.1DefinitionsofTermsSpecifictotheInstrument(SeeFig.1):attachmentarea,n—portionoftheneedlewheretheattach-mentofthesuturetakesplace.Forexample,eyed,drilled,andchannel.body,n—centralportionoftheneedleintendedtobegraspedbytheneedleholder.chordlength,n—thestraightlinedistancebetweenthetwoendsofacurvedneedle.curvature,n—theshapeoftheneedleviewedinprofile.Somecommonshapesinclude,butarenotlimitedto:straight,1⁄2curveor“ski,”1⁄8circle,1⁄4circle,3⁄8circle,1⁄2circle,5⁄8circle,andcompoundcurvature(seeFig.2).cuttingegde,n—cuttingedgesaremadeofvariousgeometricshapes,thatis,triangular,diamond,andhexagonal.Thevariousedgesmaybesharpenedbythemanufacturerde-pendingontheuserperformance.needlelength,n—thedistancemeasuredalongtheneedlecurvaturefromendtoend.needleradius,n—theradiusoftheuniformlycurvedportionorportionsoftheneedlemeasuredfromthecenterlineoftheneedlebody.needlewirediameter,n—thegageorthicknessoftheneedlewire,measuredatalocationbetweentheneedlebodyandtheattachmentarea,whereeithernoorminimalworkhastakenplace.point,n—portionoftheneedleintendedtoinitiatetissuepenetration.pointconfiguration,n—theshapeofthepoint.Somecommonpointconfigurationsinclude,butarenotlimitedto(seeFig.3):taper,trocar,blunt,spatulated,conventionalcuttingedge,reversecuttingedge,cuttingtaper,andsidecuttingneedle.swage,n—thetermusedtodescribeanyattachmentmethodthatusesmechanicalforcetocrimptheendoftheneedleandfirmlyholdthesutureinplace.2.2DefinitionsofTermsSpecifictoMechanical...