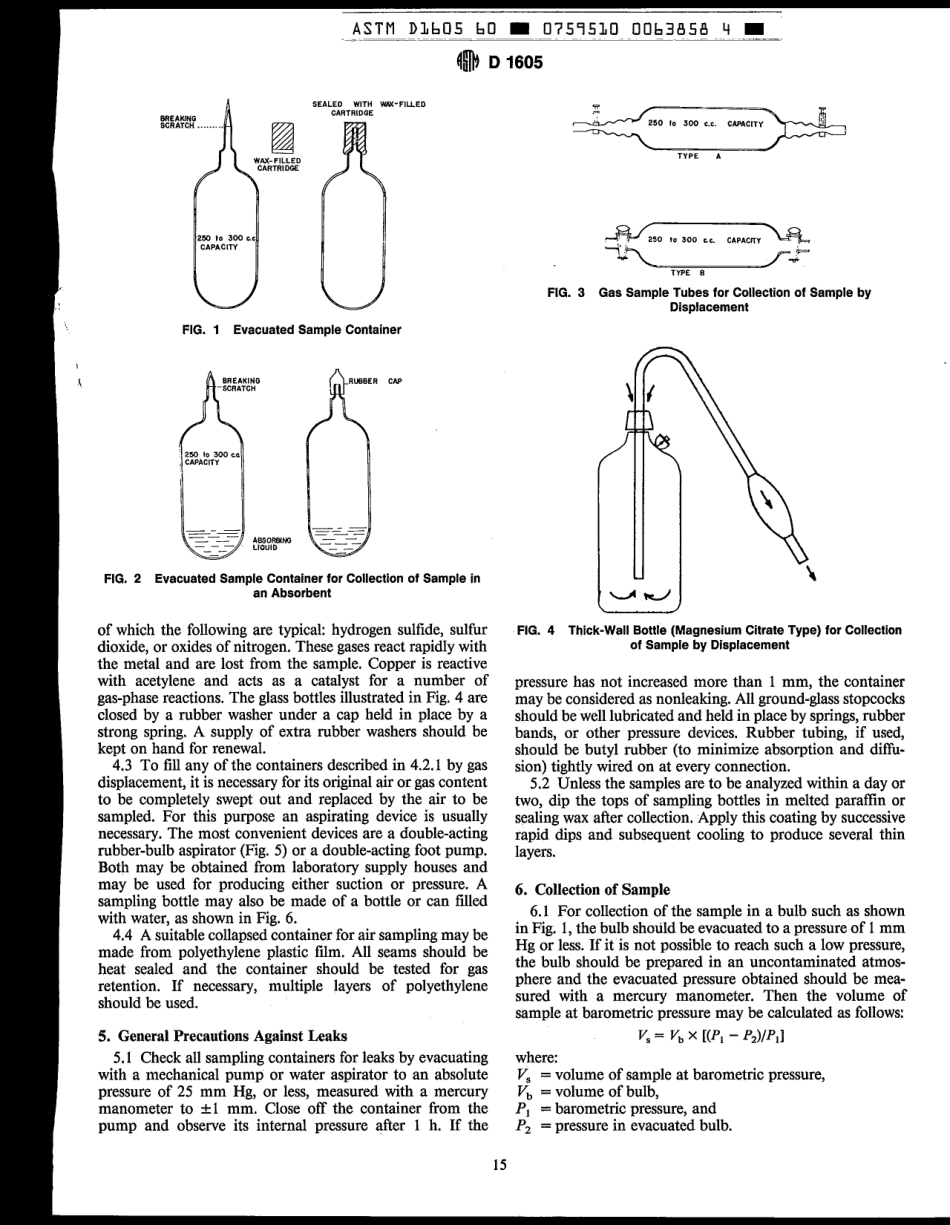
4CTt-IDesignation:D1695-60(Reapproved1990)AnAmericanNationalStandardStandardPracticesforSamplingAtmospheresforAnalysisofGasesandVapors'ThisstandardisissuedunderthefixeddesignationD1605;thenumberimmediatelyfollowingthedesignationindicatestheyearoforiginaladoptionor,inthecaseofrevision.theyearoflastrevision.Anumberinparenthesesindicatestheyearoflastreapproval.Asuperscriptepsilon(6)indicatesaneditorialchangesincethelastrevisionorreapproval.1.Scope1.ITheserecommendedpracticescoverthesamplingofatmospheresforanalysisofgasesandvapors.Twotypesofsamplingmethodsarecovered:SectionsSamplingAtmospheresWithoutConcentrationofGasesandSamplingAtmospheresWithConcentrationofGasesandVapors.............................................4to6Vapors.............................................7toi31.2Widevariationsinatmosphericcontentofextraneousgasesandvaporsprecludethepossibilityofspecifyingstandardmethodsofsamplingthatareapplicableinallcases.Definiteprincipleshave,however,beenestablishedasabasisfortheformulationofproceduresforsamplingthatareapplicableingeneralandareprobablyapplicableinmostspecificcases.Wheremodificationsoftheseproceduresarenecessary,theymaybemadebytheexerciseoftrainedjudgmentforeachcase.1.3Changesthatmaybenecessaryintheseproceduresunderspecificcircumstancesmaybemadeinanyparticularcasebymutualagreementofthepartiesconcerned.2.GeneralRequirements2.1Thefollowinggeneralrequirementsareapplicabletoallsamplingmethods:2.1.1Thesamplesmustrepresenttheconditionsexistingatthepointtaken.3.1.2Thesamplesmustbeofsufficientvolumeandmustbetakenfrequentlyenoughtopermitanaccuracyoftestingrequisiteforthedesiredobjective,asaffectedbythemethodsofanalysistobeemployed.Insomecases,however,theconcentrationofgasesorvaporstobeanalyzedwillbesolowthatitwillbeimpracticabletotakeasufficientlylargeatmosphericsample.Insuchcases,thegasesorvaporstobeanalyzedmaybeconcentratedintheatmospherepriortoanalysisofthesample.2.1.3Thesamplesmustbecollected,packed,shipped,andmanipulatedpriortoanalysisinamannerthatsafe...