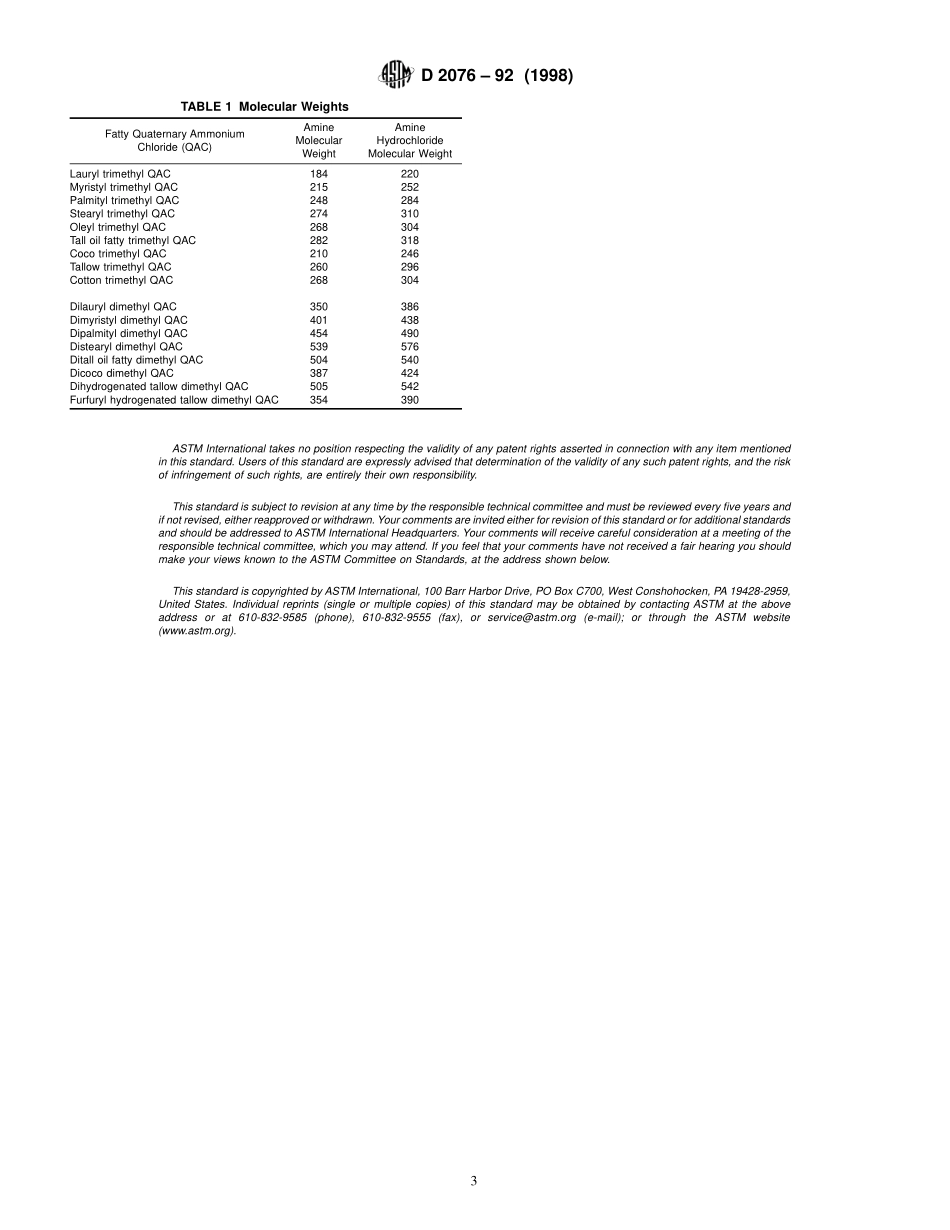
Designation:D2076–92(Reapproved1998)StandardTestMethodsforAcidValueandAmineValueofFattyQuaternaryAmmoniumChlorides1ThisstandardisissuedunderthefixeddesignationD2076;thenumberimmediatelyfollowingthedesignationindicatestheyearoforiginaladoptionor,inthecaseofrevision,theyearoflastrevision.Anumberinparenthesesindicatestheyearoflastreapproval.Asuperscriptepsilon(e)indicatesaneditorialchangesincethelastrevisionorreapproval.ThesetestmethodswerepreparedjointlybyASTMandtheAmericanOilChemists’Society.1.Scope1.1Thesetestmethodscoverthedeterminationofacidvalueandaminevalueinfattyquaternaryammoniumchlo-rides.1.2Thisstandarddoesnotpurporttoaddressallofthesafetyconcerns,ifany,associatedwithitsuse.Itistheresponsibilityoftheuserofthisstandardtoestablishappro-priatesafetyandhealthpracticesanddeterminetheapplica-bilityofregulatorylimitationspriortouse.2.ReferencedDocuments2.1ASTMStandards:D1193SpecificationforReagentWater23.Terminology3.1Definitions:3.1.1acidvalue—thenumberofmilligramsofpotassiumhydroxideneededtoneutralize1gofsample,andisusuallyduetoaminehydrochloride.3.1.2aminevalue—thenumberofmilligramsofpotassiumhydroxideequivalenttothefattyaminebasicityin1gofsample.4.Apparatus4.1ErlenmeyerFlasks,wide-mouth,alkali-resistant,boro-silicateglass,250-mLcapacity.4.2MicroBuret,10-mLcapacitygraduatedto0.02mL.5.Reagents5.1PurityofReagents—Reagentgradechemicalsshallbeusedinalltests.Unlessotherwiseindicated,itisintendedthatallreagentsshallconformtothespecificationsoftheCommit-teeonAnalyticalReagentsoftheAmericanChemicalSociety,wheresuchspecificationsareavailable.3Othergradesmaybeused,provideditisfirstascertainedthatthereagentisofsufficientlyhighpuritytopermititsusewithoutlesseningtheaccuracyofthedetermination.5.2PurityofWater—Unlessotherwiseindicated,referencestowatershallbeunderstoodtomeanreagentwaterconformingtoTypeIIofSpecificationD1193.5.3BromphenolBlueIndicatorSolution—Dissolve0.2gofbromphenolbluein100mLofmethanol,ethanol,orisopro-panol.5.4HydrochloricAcid,StandardSolution(0.1...