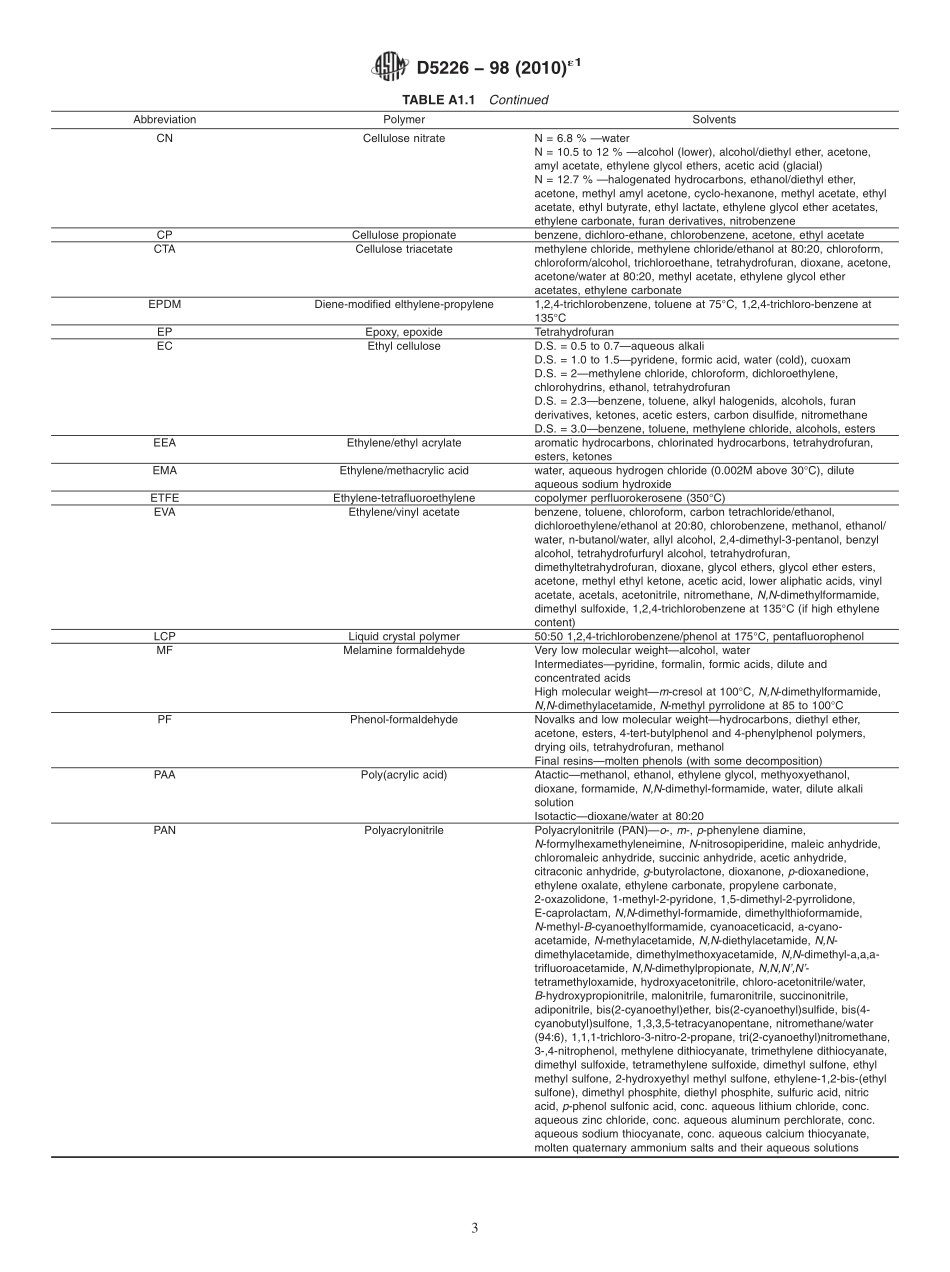
Designation:D5226−98(Reapproved2010)´1StandardPracticeforDissolvingPolymerMaterials1ThisstandardisissuedunderthefixeddesignationD5226;thenumberimmediatelyfollowingthedesignationindicatestheyearoforiginaladoptionor,inthecaseofrevision,theyearoflastrevision.Anumberinparenthesesindicatestheyearoflastreapproval.Asuperscriptepsilon(´)indicatesaneditorialchangesincethelastrevisionorreapproval.´1NOTE—ReapprovedwitheditorialchangesthroughoutinJanuary2010.1.Scope1.1Thispracticeoutlinestheparametersapplicabletothepreparationofapolymericsolution,suchassolvent,concentration,temperature,pressure,time,agitation,andheat-ingmode.1.2Theproperuseofthispracticerequiresknowledgeofsolventsandtheireffectonpolymericmaterials.1.3Thisstandarddoesnotpurporttoaddressallofthesafetyconcerns,ifany,associatedwithitsuse.Itistheresponsibilityoftheuserofthisstandardtoestablishappro-priatesafetyandhealthpracticesanddeterminetheapplica-bilityofregulatorylimitationspriortouse.NOTE1—ThereisnoknownISOequivalenttothisstandard.2.ReferencedDocuments2.1ASTMStandards:2D883TerminologyRelatingtoPlasticsD1600TerminologyforAbbreviatedTermsRelatingtoPlas-tics2.2OtherDocument:PolymerHandbook33.Terminology3.1DefinitionsareinaccordancewithTerminologyD883.3.2AbbreviationsareinaccordancewithTerminologyD1600.4.SummaryofPractice4.1Apolymersolutioncanbedescribedorpreparedusingthecellclassificationslistingtheparametersrelativetosolvatethepolymer.Thecellclassificationsarelistedinthefollowingorder:polymer,solvent,concentration,temperature,time,container,heatingmode,andagitation.4.1.1ApolymerandalistofsuggestedsolventsformakingasolutionarelistedinAnnexA1.4.1.2Table1designatestheparametersforcontainer,heat-ingmode,andtypeofagitation.NOTE2—ToillustratetheuseofthecellclassificationswithTable1,a2%solutionofpoly(vinylchloride)usingcyclohexanonewouldbewrittenas:PVC2cyclohexanone2202662402BECwhere:PVC=abbreviationofthepolymerfromAnnexA1,cyclohexanone=thesolventfromAnnexA1,20=weightofpolymerintenthsofapercent,66=temperature...