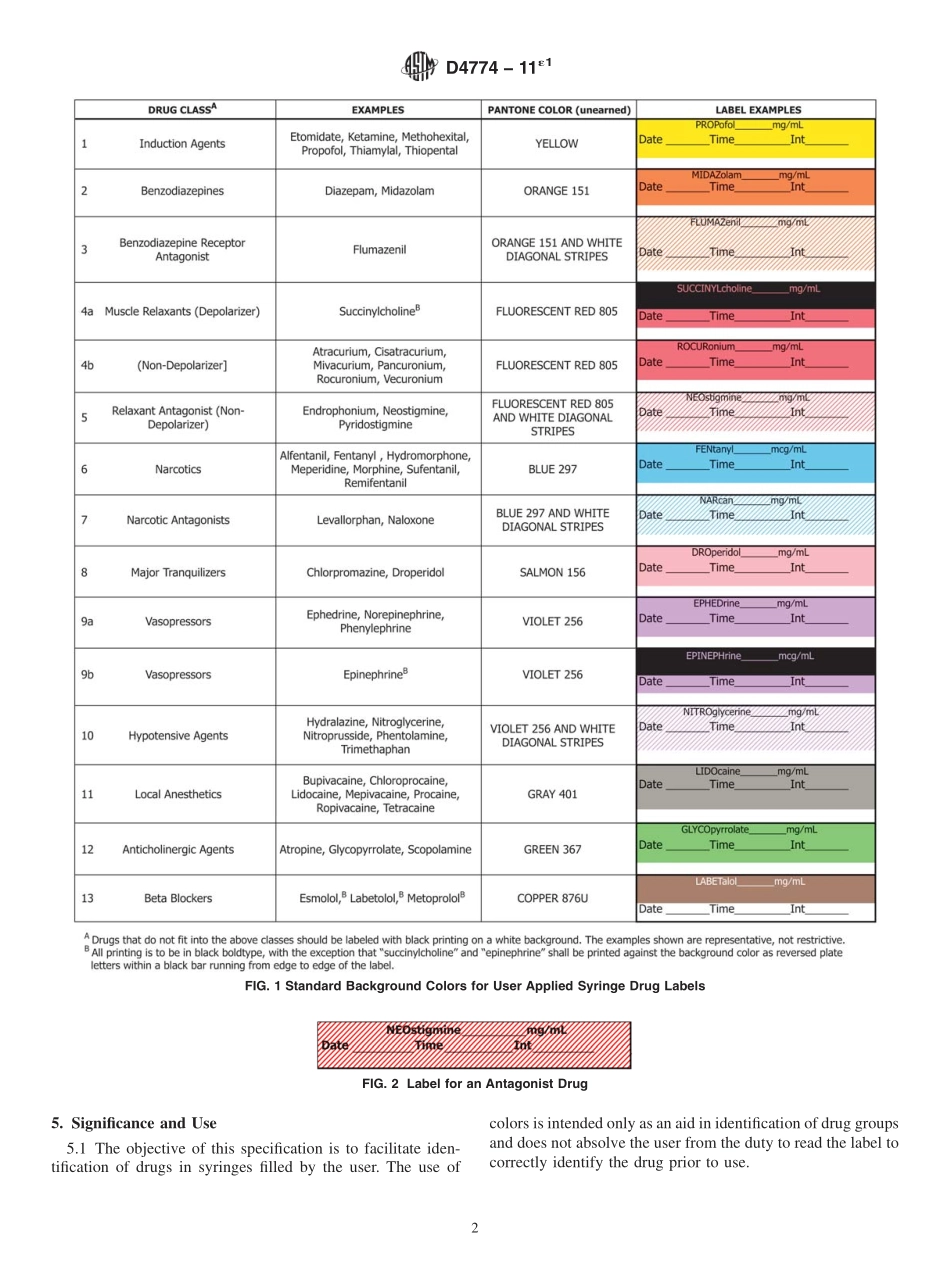
Designation:D4774−11´1StandardSpecificationforUserAppliedDrugLabelsinAnesthesiology1ThisstandardisissuedunderthefixeddesignationD4774;thenumberimmediatelyfollowingthedesignationindicatestheyearoforiginaladoptionor,inthecaseofrevision,theyearoflastrevision.Anumberinparenthesesindicatestheyearoflastreapproval.Asuperscriptepsilon(´)indicatesaneditorialchangesincethelastrevisionorreapproval.ε1NOTE—Figures1-6werecorrectededitoriallyinApril2012.1.Scope1.1Thisspecificationcoversthesize,color,pattern,andtypeusedonlabelsappliedtounlabeledsyringesfilledbytheusersortheiragentstoidentifythedrugcontent.Thisspeci-ficationisnotintendedtocoverlabelsappliedbythedrugmanufacturer.1.2ThevaluesstatedinSIunitsaretoberegardedastherecommendedvalues.Theuseofinch-poundsystemvalues,notbeingexactequivalents,mayresultinnonconformancewiththestandard.1.3Thisstandarddoesnotpurporttoaddressallofthesafetyconcerns,ifany,associatedwithitsuse.Itistheresponsibilityoftheuserofthisstandardtoestablishappro-priatesafetyandhealthpracticesanddeterminetheapplica-bilityofregulatorylimitationspriortouse.2.ReferencedDocuments2.1ASTMStandards:2D996TerminologyofPackagingandDistributionEnviron-ments2.2OtherStandard:PantoneMatchingSystem33.Terminology3.1Definitions—Generaldefinitionsforpackaginganddis-tributionenvironmentsarefoundinTerminologyD996.4.SizeandBackgroundColorRequirements4.1LabelSize—Thelabelsshallhaveanominallengthof25to35mmandawidthof10to13mm.4.2LabelBackgroundColor—ThecolorsandpatternsgiveninFig.1shallbeusedtodistinguishthesegroupsofdrugs.Thebackgroundcolorshallnotinterferewiththeabilityoftheusertowriteinformationonthelabel.4.2.1Antagonists—Todenoteanantagonist,1-mmwidediagonalstripesoftheagonistcoloralternatingwitha1-mmwidewhitestripeshallbeused.Thestripesshallrunfromthelowerlefttotheupperrightatanangleofapproximately45°tothelongaxisofthelabel.Thenameofanantagonistdrugshallappearinthecenterofthelabelandthestripingshallbeomittedbehindandbelowthename(seeFig.2).1Thisspecificationisundertheju...