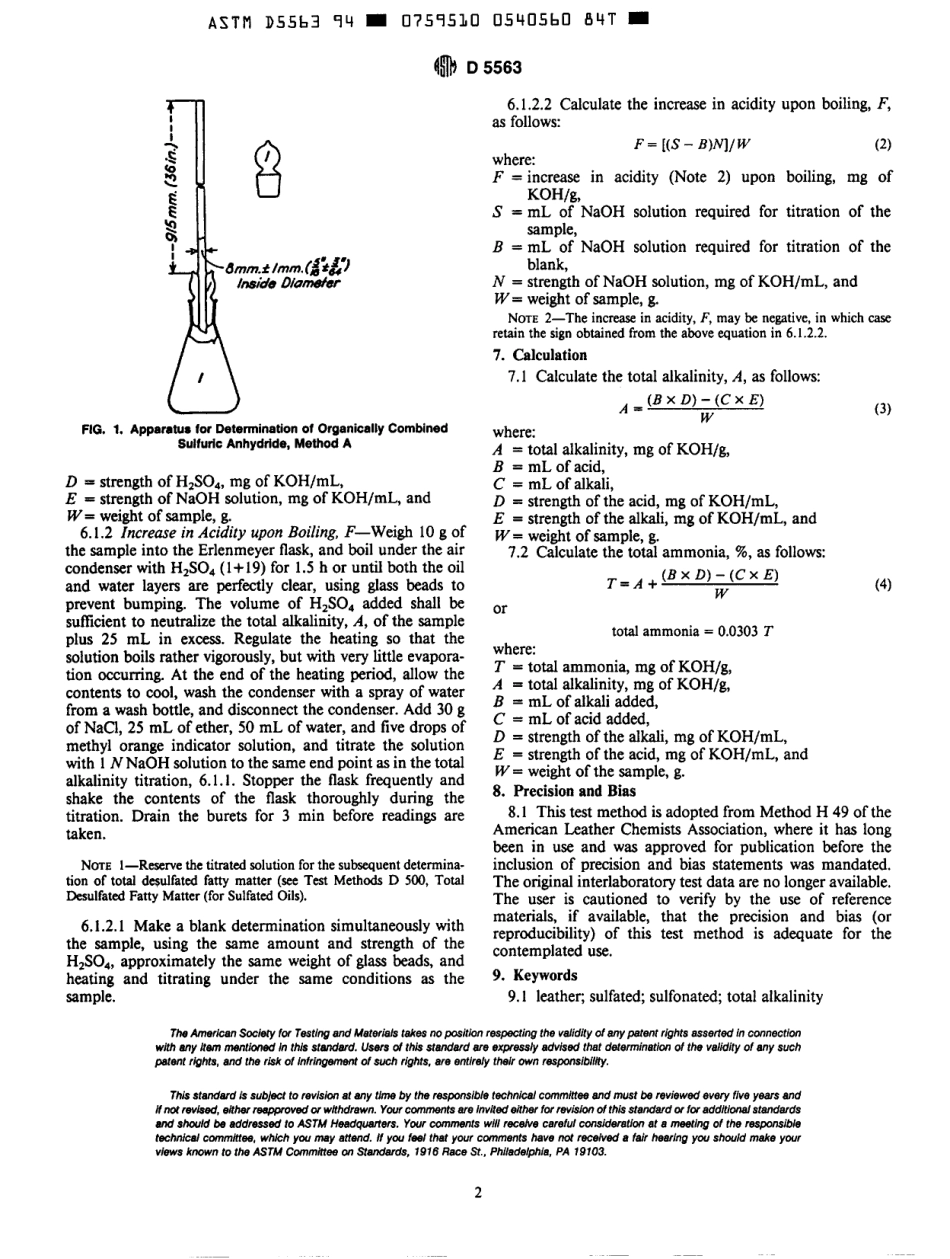
ASTMD55639407595300540559028AMERICANSOCIETYFORTESTINGANDMATERIALS1916RaceSt.Philadelphia,Pa19103ReprintedfromtheAnnualBookofASTMStandards.CopyrightASTMIfnotlistedinthecurrentcombinedindexwillappearintheneledition4ibDesignation:D5563-94StandardTestMethodforDeterminationoftheTotalAlkalinityofSulfonatedorSulfatedOils'Thisstandardisissuedunderthefixeddesignation05563;thenumberimmediatelyfollowingthedesignationindicatestheyearoforiginaladoptionor,inthecaseofrevision,theyearoflastrevision.Anumberinparenthesesindicatestheyearoflastreapproval.Asuperscriptepsilon(t)indicatesaneditorialchangesincethelastrevisionorreapproval.1.Scope1.1Thistestmethodcoversdeterminationofthetotalalkalinityofsulfonatedorsulfatedoils.1.2ThevaluesstatedinSIunitsaretoberegardedasthestandard.1.3Thisstandarddoesnotpurporttoaddressallofthesafetyconcerns,ifany,associatedwithitsuse.Itistheresponsibilityoftheuserofthisstandardtoestablishappro-priatesafetyandhealthpracticesanddeterminetheapplica-bilityofregulatorylimitationspriortouse.2.ReferencedDocuments2.1ASTMStandard:D500TestMethodsforChemicalAnalysisofSulfonatedandSulfatedOils22.2ALCAMethod:H49MethodforTotalAlkalinityandAmmonia33.SignificanceandUse3.IThistestmethodisintendedtocoverdeterminationofthetotalalkalinityexistinginasampleofsulfonatedorsulfatedoil,orboth,bytitratingawatersolutionofthesamplewithmineralacidinthepresenceofmethylorangeastheindicator.Thistestmethodcoversdeterminationofthetotalalkalinityofthefixedalkali,ammonia,andtriethanolamineboundassoap,freealkali,andthealkalinityoftitratablealkalinesalts,butnotthatofnontitratablealkalinesalts.4.Apparatus4.1Theapparatusrequiredconsistsofaglassflaskpro-videdwithaglassstopperandanaircondenser.Theconnectionbetweentheflaskandthecondensershallbeagroundjoint.Perforatedglassbeadsshallbeusedtopreventbumping.4.1.1Flask-AnErlenmeyerflask(Fig.I)madeofaborosilicateglass,havingacapacityofapproximately300mLandprovidedwithaglassstopper.4.1.2Condenser,consistingofaglasstube,915mm(36in.)inlengthand8mm(Y16in.)...