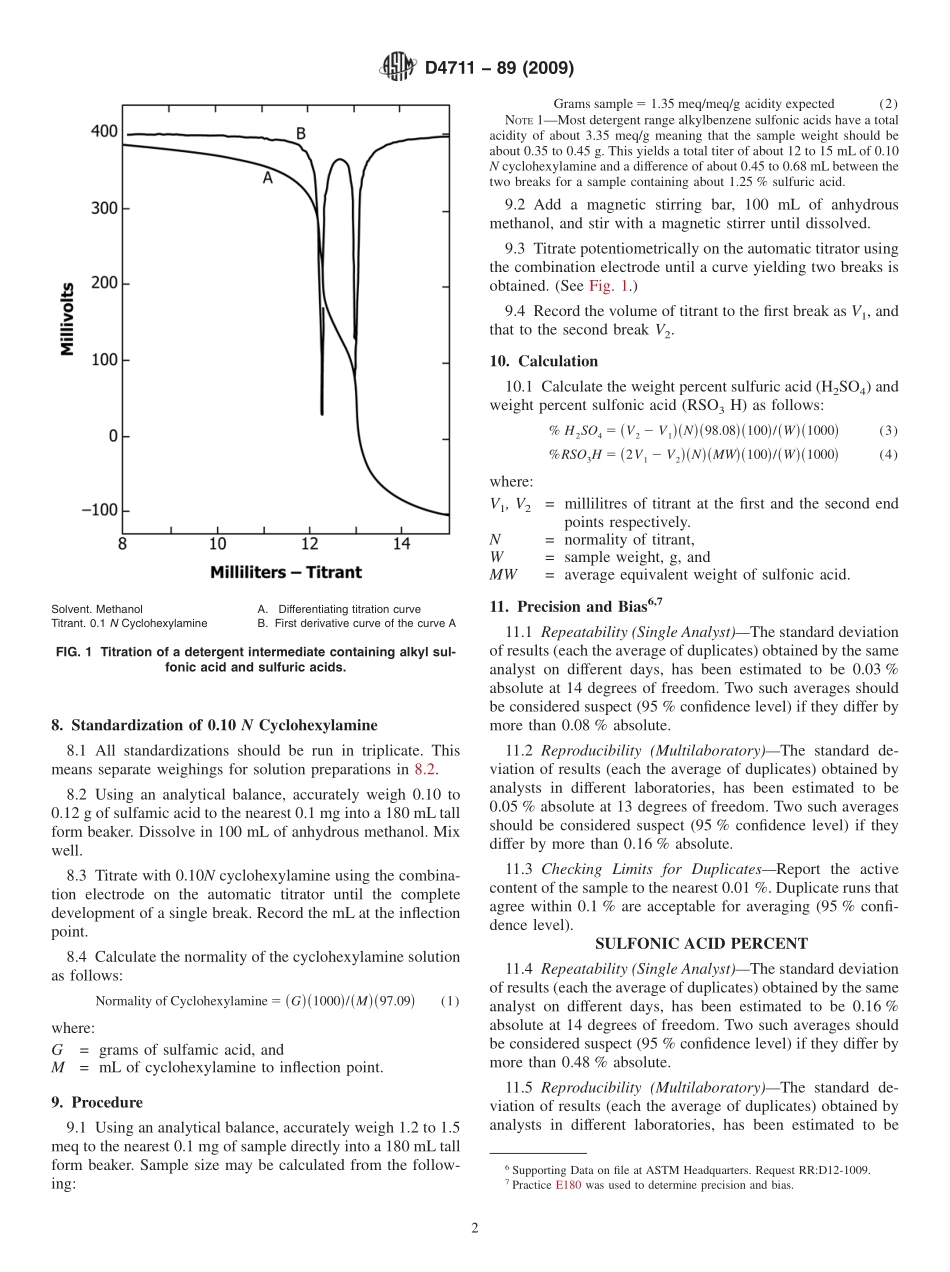
Designation:D4711−89(Reapproved2009)StandardTestMethodforSulfonicandSulfuricAcidsinAlkylbenzeneSulfonicAcids1ThisstandardisissuedunderthefixeddesignationD4711;thenumberimmediatelyfollowingthedesignationindicatestheyearoforiginaladoptionor,inthecaseofrevision,theyearoflastrevision.Anumberinparenthesesindicatestheyearoflastreapproval.Asuperscriptepsilon(´)indicatesaneditorialchangesincethelastrevisionorreapproval.1.Scope1.1Thistestmethodisapplicabletothedeterminationofsulfonicandsulfuricacidsinbranchedandlinearalkylbenzenesulfonicacidsusedasintermediatesinsyntheticdetergents.1.2ThevaluesstatedinSIunitsaretoberegardedasstandard.Nootherunitsofmeasurementareincludedinthisstandard.1.3Thisstandarddoesnotpurporttoaddressallofthesafetyconcerns,ifany,associatedwithitsuse.Itistheresponsibilityoftheuserofthisstandardtoestablishappro-priatesafetyandhealthpracticesanddeterminetheapplica-bilityofregulatorylimitationspriortouse.MaterialSafetyDataSheetsareavailableforreagentsandmaterials.Reviewthemforhazardspriortousage.2.ReferencedDocuments2.1ASTMStandards:2D459TerminologyRelatingtoSoapsandOtherDetergentsE180PracticeforDeterminingthePrecisionofASTMMethodsforAnalysisandTestingofIndustrialandSpe-cialtyChemicals(Withdrawn2009)33.SummaryofTestMethod3.1Amethanolicsolutionofthesampleistitratedwithcyclohexylamineinmethanoltoyieldapotentiometriccurve.(SeeFig.1.)Thefirstinflectionrepresentstheneutralizationofstrongacids,suchassulfonicsandalkylsulfurics,andthefirsthydrogenofsulfuricacid.Thesecondinflectionrepresentstheneutralizationofthesecondhydrogenofsulfuricacid.Theamountofsulfonicacidiscalculatedbasedonthetitrantvolumeofthefirstinflectionminusthatbetweenthetwoinflections.Theamountofsulfuricacidmeanwhileiscalcu-latedfromthetitrantvolumebetweenthetwoinflections,whichisequivalenttotheamountofbaserequiredforneutralizationofthebisulfateanion.4.SignificanceandUse4.1Alkylbenzenesulfonicacidsareimportantintermediatesinthesyntheticdetergentindustryandaredefinedunder“alkylbenzenesulfonate”i...