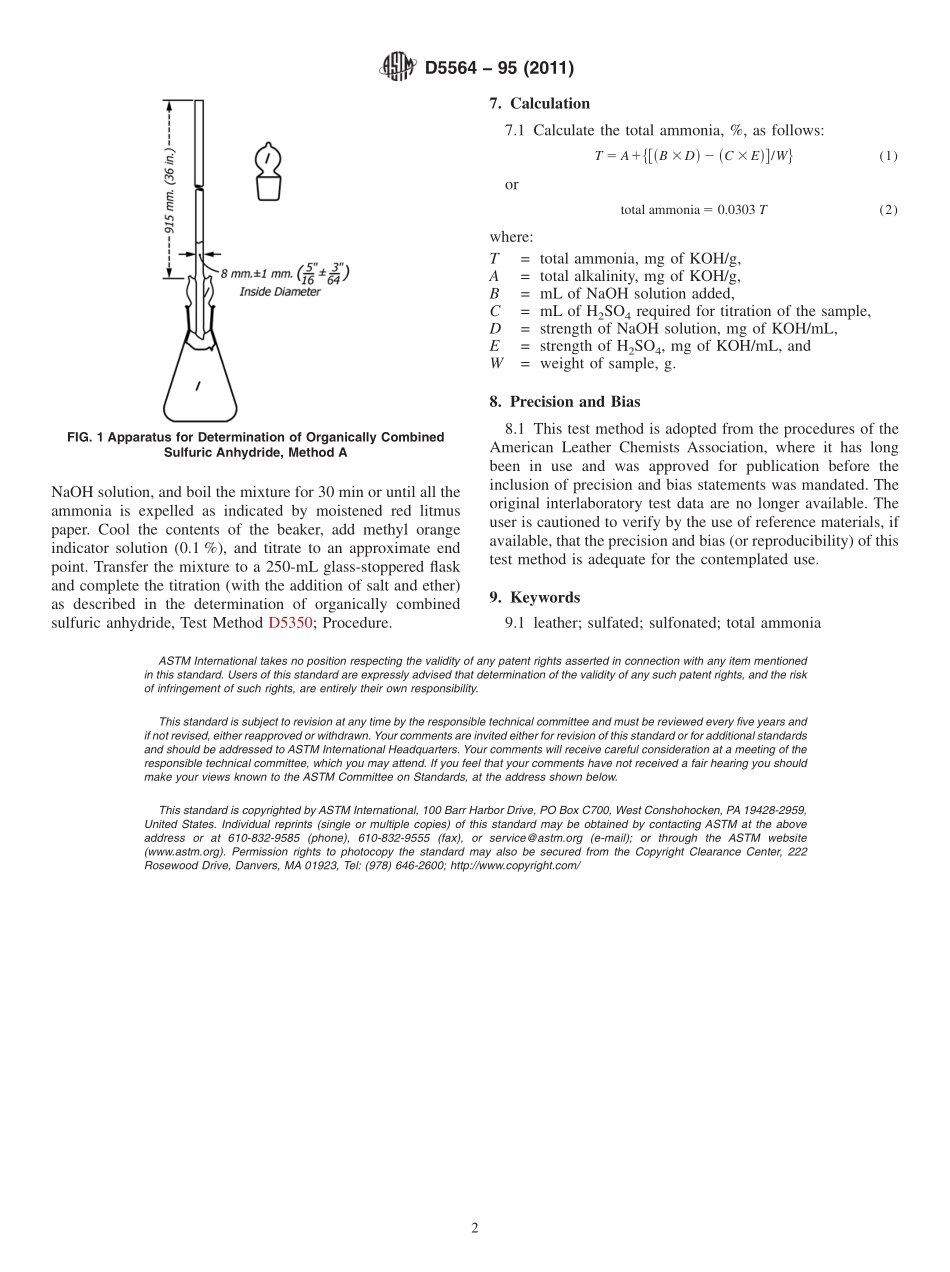
Designation:D5564−95(Reapproved2011)StandardTestMethodforDeterminationoftheTotalAmmoniaContainedinSulfonatedorSulfatedOils1ThisstandardisissuedunderthefixeddesignationD5564;thenumberimmediatelyfollowingthedesignationindicatestheyearoforiginaladoptionor,inthecaseofrevision,theyearoflastrevision.Anumberinparenthesesindicatestheyearoflastreapproval.Asuperscriptepsilon(´)indicatesaneditorialchangesincethelastrevisionorreapproval.1.Scope1.1Thistestmethodcoversdeterminationofthetotalammoniacontainedinsulfonatedorsulfatedoils.1.2ThevaluesstatedinSIunitsaretoberegardedasthestandard.1.3Thisstandarddoesnotpurporttoaddressallofthesafetyconcerns,ifany,associatedwithitsuse.Itistheresponsibilityoftheuserofthisstandardtoestablishappro-priatesafetyandhealthpracticesanddeterminetheapplica-bilityofregulatorylimitationspriortouse.2.ReferencedDocuments2.1ASTMStandards:2D5350TestMethodforDeterminationofOrganicallyCom-binedSulfuricAnhydridebyTitration,TestMethodA3.SignificanceandUse3.1Thistestmethodofanalysisisintendedtodeterminethetotalammoniainasampleofsulfonatedorsulfatedoil,orboth,byboilingawatersolutionofthesamplewithexcessalkalianddeterminingbytitrationthelossinalkaliaftertheboiling.4.Apparatus4.1Theapparatusrequiredconsistsofaglassflaskprovidedwithaglassstopperandanaircondenser.Theconnectionbetweentheflaskandthecondensershallbeagroundjoint.Perforatedglassbeadsshallbeusedtopreventbumping.4.1.1Flask—AnErlenmeyerflask(Fig.1)madeofaboro-silicateglass,havingacapacityofapproximately300mLandprovidedwithaglassstopper.4.1.2Condenser,consistingofaglasstube,915mm(36in.)inlengthand8mm(5⁄16in.)inoutsidediameter.ThelowerendofthetubeshallbeflaredandgroundtofitthemouthoftheErlenmeyerflask.4.1.3GlassBeads—Perforatedglassbeads,madeofchemi-callyresistantglass,approximately4mm(5⁄32in.)indiameter.Beforeusing,theglassbeadsshallbeboiledthoroughlyinseveralportionsofwateroruntilthewashwaterreactsneutraltoamethylorangeindicator.5.Reagents5.1EthylEther.5.2MethylOrangeIndicatorSolution(1g/L)—Dissol...