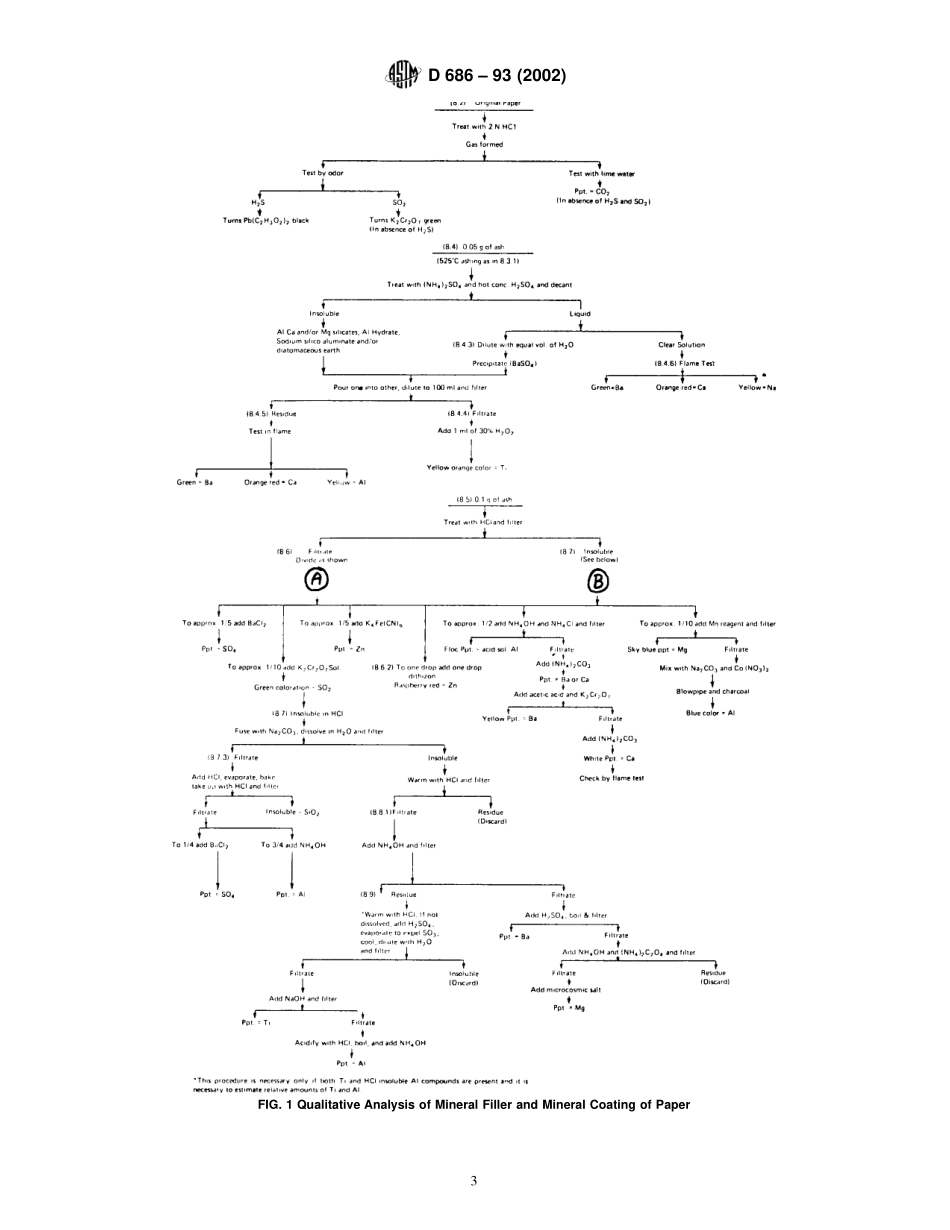
Designation:D686–93(Reapproved2002)AnAmericanNationalStandardStandardTestMethodsofQualitativeExaminationofMineralFillerandMineralCoatingofPaper1ThisstandardisissuedunderthefixeddesignationD686;thenumberimmediatelyfollowingthedesignationindicatestheyearoforiginaladoptionor,inthecaseofrevision,theyearoflastrevision.Anumberinparenthesesindicatestheyearoflastreapproval.Asuperscriptepsilon(e)indicatesaneditorialchangesincethelastrevisionorreapproval.1.Scope1.1Thesetestmethodscovertwoproceduresforthequali-tativedeterminationandidentificationofthemineralconstitu-entsoffilledandcoatedpapers.1.2Duetothesimilarityinchemicalcompositionandphysicalsizeandshapeofsomeofthevariouspossibleconstituentscontainedinagivenpaperspecimen,morepre-cise,quantitativemethodsmayattimesberequiredforpositiveidentification.1.3Itisrecommendedthatonebecomethoroughlyfamiliarwiththesetestmethodsbyanalyzingpapersamplesofknownmineralcomponentcontent.1.4Thetestmethodsappearasfollows:SectionsMethodA—ChemicalAnalysis4to11MethodB—MicroscopicalIdentification2to191.5Thisstandarddoesnotpurporttoaddressallofthesafetyconcerns,ifany,associatedwithitsuse.Itistheresponsibilityoftheuserofthisstandardtoestablishappro-priatesafetyandhealthpracticesanddeterminetheapplica-bilityofregulatorylimitationspriortouse.NOTE1—ThesetestmethodsaretechnicallyequivalenttoTAPPIT421–83.2.ReferencedDocuments2.1ASTMStandards:D585PracticeforSamplingandAcceptingaSingleLotofPaper,Paperboard,Fiberboard,andRelatedProducts2D586TestMethodforAshinPulp,Paper,andPaperProducts2D921TestMethodforTitaniumDioxideinPaper3D1030TestMethodforFiberAnalysisofPaperandPaperboard22.2TAPPIStandards:T401Fiberanalysisofpaperandpaperboard4T438Zincandcadmiuminpaperandpigments4TestMethodA—QualitativeChemicalAnalysis3.SignificanceandUse3.1Qualitativechemicalanalysesofthemineralcomponentofapaperspecimen,TestMethodA,servetoidentifytheionsofanysuchminerals.Theresultsmaythenbeinterpretedintermsofthemineralsthemselves.Directidentificationofsomeofthesemineralsorthe...