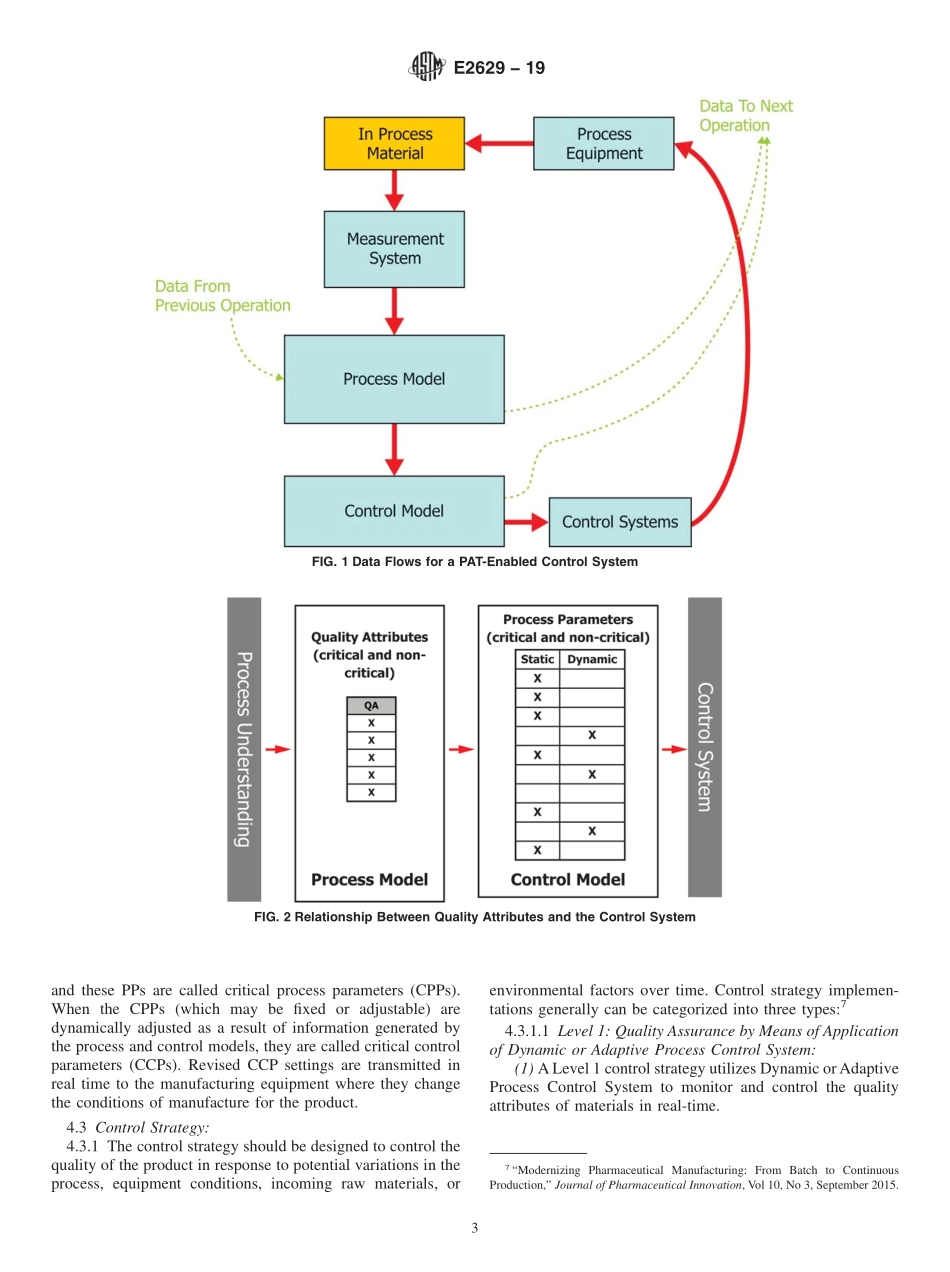
Designation:E2629−19StandardGuideforVerificationofProcessAnalyticalTechnology(PAT)EnabledControlSystems1ThisstandardisissuedunderthefixeddesignationE2629;thenumberimmediatelyfollowingthedesignationindicatestheyearoforiginaladoptionor,inthecaseofrevision,theyearoflastrevision.Anumberinparenthesesindicatestheyearoflastreapproval.Asuperscriptepsilon(´)indicatesaneditorialchangesincethelastrevisionorreapproval.1.Scope1.1Thisguidedescribestheverificationofprocessanalyti-caltechnology(PAT)enabledcontrolsystemsusingascience-andrisk-basedapproach.Itestablishesprinciplesfordetermin-ingthescopeandextentofverificationactivitiesnecessarytoensurethatthePAT-enabledcontrolsystemisfitforpurpose,properlyimplemented,andfunctionsasexpected.1.2Inthisguide,aPAT-enabledcontrolsystemisconsid-eredtobethesystemthatadjuststhemanufacturingprocessusingtimelymeasurements(thatis,duringprocessing)ofattributesofrawandin-processmaterialstodeterminere-sponsesthatassuretheprocessremainswithinspecifiedboundariesandminimizesvariabilityintheoutputmaterial.TheoverallaimofthePAT-enabledcontrolsystemistoensureproductquality.ThePAT-enabledcontrolsystemofamanu-facturingprocessprovidesthecapabilitytodeterminethecurrentstatusoftheprocessanddrivetheprocesstoensuretheoutputmaterialhasthedesiredqualitycharacteristics.Thecontrolsystemshouldbeabletorespondtoprocessvariationsinatimelymanner,providingcorrectionsthatensurethattheprocessfollowsthedesiredprocesstrajectorytoreachthedesiredoutcome.PAT-enabledcontrolsystemsmayusepro-cessmodelsbasedonfirstprinciplesunderstandingorempiri-calmodelsderivedfromexperimentalinvestigationsorboth.Inadditiontoautomatedcontrols,aPAT-enabledcontrolsystemmayincludecomponentswherethereismanualinter-vention.1.3PrinciplesdescribedinthisguidemaybeappliedregardlessofthecomplexityorscaleofthePAT-enabledcontrolsystemorwhetherappliedtobatchorcontinuousprocessing,orboth.TheintentionofthisstandardistodescribeandsupporttheimplementationofaPATenabledControlStrategy,asdescribedinICHQ8(R2).1.4T...