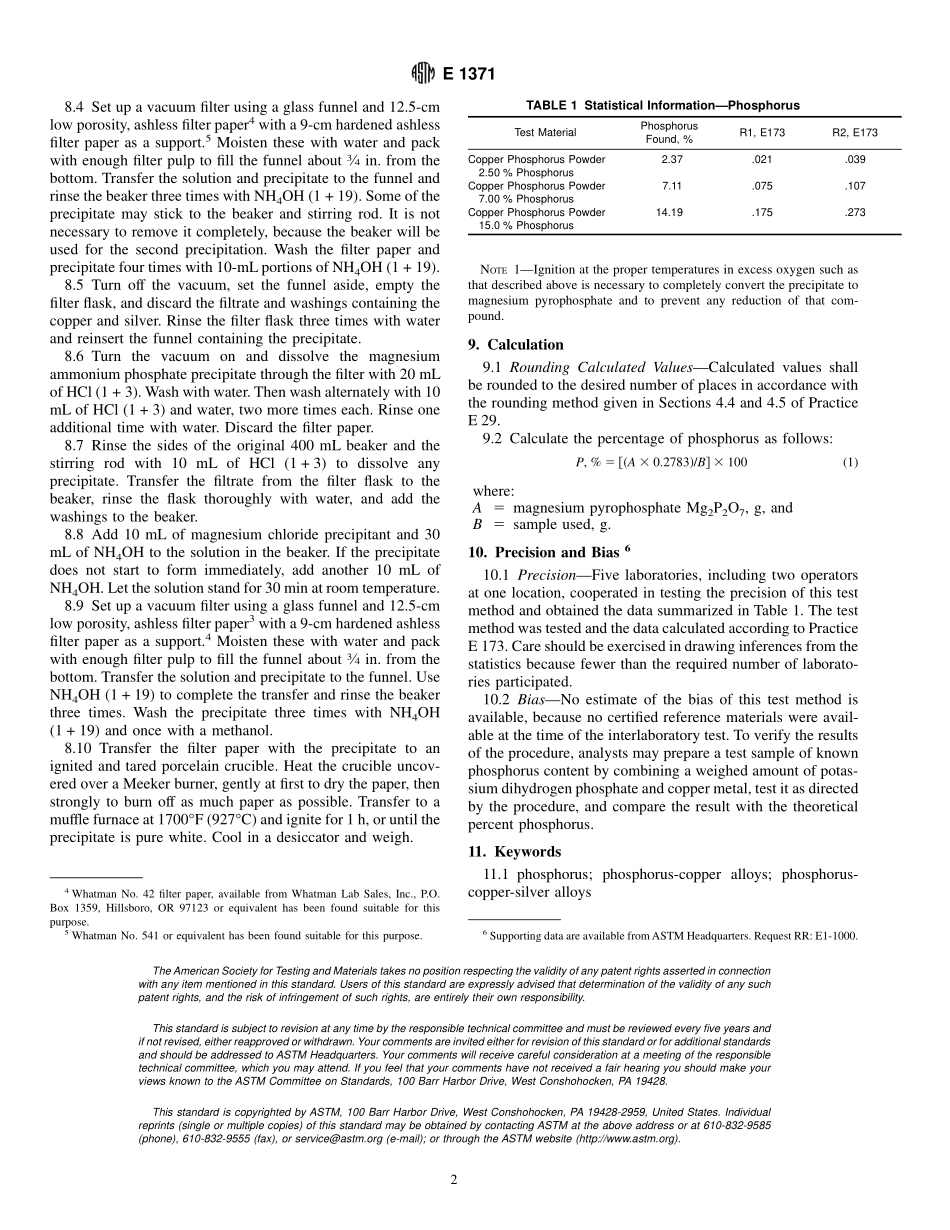
Designation:E1371–90(Reapproved1999)StandardTestMethodforGravimetricDeterminationofPhosphorusinPhosphorus-CopperAlloysorPhosphorus-Copper-SilverAlloys1ThisstandardisissuedunderthefixeddesignationE1371;thenumberimmediatelyfollowingthedesignationindicatestheyearoforiginaladoptionor,inthecaseofrevision,theyearoflastrevision.Anumberinparenthesesindicatestheyearoflastreapproval.Asuperscriptepsilon(e)indicatesaneditorialchangesincethelastrevisionorreapproval.1.Scope1.1Thistestmethodcoversthegravimetricdeterminationofphosphorusinphosphorus-copperorphosphorus-copper-silveralloyscontaining1to15%phosphorus.1.2Thisstandarddoesnotpurporttoaddressthesafetyconcerns,ifany,associatedwithitsuse.Itistheresponsibilityoftheuserofthisstandardtoestablishappropriatesafetyandhealthpracticesanddeterminetheapplicabilityofregulatorylimitationspriortouse.2.ReferencedDocuments2.1ASTMStandards:E29PracticeforUsingSignificantDigitsinTestDatatoDetermineConformancewithSpecifications2E173PracticeforConductingInterlaboratoryStudiesofMethodsforChemicalAnalysisofMetals3E255PracticeforSamplingCopperandCopperAlloysforDeterminationofChemicalComposition33.SummaryofTestMethod3.1Afterdissolutionofthesampleinnitricacid,phospho-rusisprecipitatedwithammoniacalmagnesiumchloride.Magnesiumammoniumphosphateisseparatedbyfiltrationandredissolvedwithdilutehydrochloricacid.Phosphorusisrepre-cipitatedwithammoniacalmagnesiumchloride,thenfiltered,ignited,andweighedasmagnesiumpyrophosphate.4.SignificanceandUse4.1Thistestmethodforthechemicalanalysisofmetalsandalloysisprimarilyintendedtotestsuchmaterialsforcompli-ancewithcompositionalspecifications.Itisassumedthatallwhousethismethodwillbetrainedanalystscapableofperformingcommonlaboratoryproceduresskillfullyandsafely.Itisexpectedthattheworkwillbeperformedinaproperlyequippedlaboratory.5.Interferences5.1Silveriscomplexedasdiamminesilver(I).Copperiscomplexedastetraminecopper(II).5.2Whenpresent,tininterferesbyforminginsolublestan-nicphosphate.Otherelementswhichforminsolublepho...